ATMO551A FALL 2010 LATENT HEAT OF FUSION AND VAPORIZATION
ATMO 551A WWWATMOARIZONAEDUSTUDENTSCOURSELINKSFALL10ATMO551AADIABATICLAPSERATEDOC DRY ADIABATIC TEMPERATURE LAPSE RATE ASATMO551A FALL 2010 ESTIMATING SINKING VELOCITY OF AIR IN
ATMO551A FALL 2010 LATENT HEAT OF FUSION AND VAPORIZATION
Latent heats
ATMO551a Fall 2010
Latent heat of fusion and vaporization
Latent heat is the energy change associated with the phase change of a material between gas, liquid and solid. The latent heat is written as L and given in J/kg in mks units. It literally means how much heat energy must be added to a mass of material to convert its phase such as from solid to liquid (melt), liquid to vapor (evaporate) or solid to vapor (sublimate).
DQ = m L
When the transition occurs the other way such as vapor to liquid, the latent heat defines how much energy is released from the mass into the environment.
+540
cal/gm
+80
cal/gm
-540
cal/gm
-80
cal/gm
-620
cal/gm
+620
cal/gm
Surrounding
air
warms
Surrounding
air
cools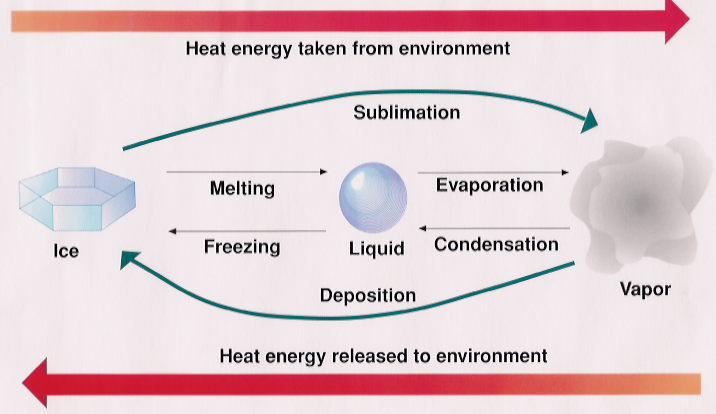
Note that 1 calorie = 4.1868 J.
Examples of Latent heats of various materials
|
Substance |
Latent Heat Fusion kJ/kg |
Latent Heat Fusion J/mole |
Melting Point °C |
Latent Heat Vaporization kJ/kg |
Boiling Point °C at 1013 mb |
|
Alcohol, ethyl |
108 |
|
-114 |
855 |
78.3 |
|
Ammonia |
339 |
|
-75 |
1369 |
-33.34 |
|
Carbon dioxide |
184 |
|
-57 |
574 |
-78 |
|
Helium |
|
|
|
21 |
-268.93 |
|
Hydrogen |
58 |
|
-259 |
455 |
-253 |
|
Lead |
24.5 |
|
372.3 |
871 |
1750 |
|
Methane |
58.7 |
|
-182. |
510 |
-161.6 °C |
|
Nitrogen |
25.7 |
|
-210 |
200 |
-196 |
|
Oxygen |
13.9 |
|
-219 |
213 |
-183 |
|
Water |
334 |
|
0 |
2500 (at 0oC) |
100 |
The magnitude of the latent heat is a measure of how strongly bound the molecules are to one another in the liquid and solid states. Note in the table above that water has the highest latent heat of vaporization. The next highest is ammonia, NH3, which is similar in many ways to water, H2O. Both are asymmetrical molecules with large permanent electric dipole moments that make the molecules readily and tightly bind to one another.
Molar form of the latent heat reveals more about the binding energy.
Heat required to melt and boil some water
Take 5 grams of ice initially at -20oC. How much energy does it take to raise the water molecules in the ice to a temperature of 100oC and fully vaporize the water molecules?
add heat to raise the temperature of the ice to 0oC.
add heat to melt the ice
add heat to raise the liquid water from 0oC to 100oC
add heat to vaporize the liquid water and convert the molecules from liquid to gas phase
To make these calculations, we need to know the specific heats.
|
Material |
Cp (J/g/K) |
Cp,m (J/mol/K) |
CV,m (J/mol/K) |
|
Water vapor (100 °C) |
2.080 |
37.47 |
28.03 |
|
Water liquid (25 °C) |
4.1813 |
75.327 |
74.53 |
|
Water ice (-10 °C) |
2.050 |
38.09 |
|
Given what we know about specific heats, what can we say about why these specific heats differ?
Tags: atmo551a fall, fusion, latent, vaporization, atmo551a
- HOME OFFICE ONE NATIONWIDE PLAZA • COLUMBUS OHIO 43215
- 3 TELEPHONE TALK PAGE 19 1 TELEPHONE NUMBERS
- CONVERGENCIA EN LAS REGIONES ESPAÑOLAS CAMBIO TÉCNICO EFICIENCIA Y
- SUBMITTING A NAIROBI WORK PROGRAMME ACTION PLEDGE AN ACTION
- VERZOEK TOT BESPREKING JEUGDBESCHERMINGSTAFEL REGIO MIDDEN IJSSEL OOST
- TEMA LA SOLIDARIDAD QUE NACE DE LA FE
- AMT FÜR LEBENSMITTELSICHERHEIT UND TIERGESUNDHEIT 2 ANALISI DEI RISCHI
- CHEQUE PRINTER SELF ACCREDITATION PROGRAM THE SELF ACCREDITATION PROGRAM
- FOR IMMEDIATE RELEASE FOR MORE INFORMATION CONTACT JUNE 2
- CENTAR ZA SOCIJALNU SKRB VELIKA GORICA TRG K TOMISLAVA
- CONTRATO DE MODIFICACIÓN DEL REGLAMENTO DE GESTIÓN DE FONDOS
- F02HVCBA6 HVCB FIRSTPOLETOCLEAR TASK FORCE THE HVCB FIRSTPOLETOCLEAR
- DANH SÁCH BAN LỄ TANG ĐỒNG CHÍ THƯỢNG TƯỚNG
- 4 CHAPITRE 2 ENTREPRISES ET PRODUCTION I LA
- DECRETO 1761 DE 1990 (AGOSTO 2) POR EL CUAL
- RESOURCE REQUEST (ICS 213 RR) ADAPTED FOR NM SEOC
- 64 INTRODUCCION CON LA CRECIENTE DEMANDA MUNDIAL DE METALES
- MINISTRSTVO ZA KMETIJSTVO GOZDARSTVO IN PREHRANO DUNAJSKA 22 1000
- TIPS FOR MAKING ART WITH YOUNG PEOPLE THE YOUNG
- UN SISTEMA TECNOLÓGICO DE GESTIÓN DE ENCUESTAS DE SATISFACCIÓN
- ALICJA CZERKAWSKA UNIWERSYTET WROCŁAWSKI STUDIUM DOKTORANCKIE PRZYDATNOŚĆ KLASYFIKACJI PROBLEMÓW
- PCIPD35 PAGE 0 WIPO E PCIPD35 ORIGINAL ENGLISH DATE
- U LSTER SQUASH PRESEASON OPEN BELFAST BOAT CLUB FRIDAY
- NA OSNOVU ČLANA 14 STAV 2 UREDBE O GENERALNOM
- SECTION IX COLORS 1042 GENERAL A FLAGS
- CONTEXT ES TRACTA DE DOS GRUPS DE 2N D’ESO
- PRESENTACIÓ DE LA BIOGRAFIA DEL BISBE HUIX DÉU VOS
- REVIEW SEPT 2014 CLEAR DESK SCREEN POLICY INTRODUCTION
- DLA V ROKU WYDZ INŻ ŚRODOWISKA SPECJ KLIMATYZ
- SAMPLE FOCUS GROUP RESEARCH – INFORMATION SHEET INSTRUCTIONS STATEMENTS
HORARIO DE LA PRIMAVERA DEL 2013 CURSO DE MÁSTER
 PODPORA TRVALÉ UDRŽITELNOSTI DOSTUPNOSTI A ZKVALITŇOVÁNÍ SOCIÁLNÍCH SLUŽEB NA
PODPORA TRVALÉ UDRŽITELNOSTI DOSTUPNOSTI A ZKVALITŇOVÁNÍ SOCIÁLNÍCH SLUŽEB NA TÍTULO DEL ARTÍCULO PAPER TITLE AUTOR 1 ULTIMO TÍTULO
TÍTULO DEL ARTÍCULO PAPER TITLE AUTOR 1 ULTIMO TÍTULO NÁZEV SEMINÁRNÍ PRÁCE FAKULTA INFORMATIKY A MANAGEMENTU UNIVERZITA HRADEC
NÁZEV SEMINÁRNÍ PRÁCE FAKULTA INFORMATIKY A MANAGEMENTU UNIVERZITA HRADEC A CHARGING MODEL FOR SESSIONS ON THE INTERNET NADIA
A CHARGING MODEL FOR SESSIONS ON THE INTERNET NADIA PREPOSITIONS IN L1 & L2 SHARON ARMONLOTEM PREPOSITIONS IN
PREPOSITIONS IN L1 & L2 SHARON ARMONLOTEM PREPOSITIONS INHUMANITARIAN ASSISTANCE STRATEGY 2014 – PRELIMINARY DISBURSEMENT OUTLOOK TIMEFRAME
DRUG THERAPY INSTEAD OF CRIMINALIZING DRUG ADDICTS UNITED NATIONS
 ZAŁĄCZNIK NR 6 DO OGŁOSZENIA (PIECZĘĆ ADRESOWA WYKONAWCY) WYKAZ
ZAŁĄCZNIK NR 6 DO OGŁOSZENIA (PIECZĘĆ ADRESOWA WYKONAWCY) WYKAZAPPENDIX B INSURANCE COMPANIES FOUNDED IN CADIZ 1790–1814 DOC
ANNEX 2 TEHNIČKA SPECIFIKACIJA I TEHNIČKA PONUDA OTVORENI
 COMUNICADO DE PRENSA LA OFERTA UNE LAS DIFERENTES APLICACIONES
COMUNICADO DE PRENSA LA OFERTA UNE LAS DIFERENTES APLICACIONESFONDOS DE EMPLEADOS CONCEPTO NO 032629 DEL 4 DE
 S OP 420 METH LAB WASTES CONTENTS 1 INTRODUCTION
S OP 420 METH LAB WASTES CONTENTS 1 INTRODUCTION 43 POKUS O HISTORICKOU STUDII NAŠEHO POMÝLENÍ ÚVOD VYRŮSTAL
43 POKUS O HISTORICKOU STUDII NAŠEHO POMÝLENÍ ÚVOD VYRŮSTALDDÑA VECINOA DE CP DOMICILIADOA EN
NAZIV KOLEGIJA JEZIČNE VJEŽBE IZ SLOVENSKOG JEZIKA 1 NASTAVNICA
 TITLE (TIMES NEW ROMAN 14 PT BOLD CENTER ALIGNED)
TITLE (TIMES NEW ROMAN 14 PT BOLD CENTER ALIGNED) R ADA MIEJSKA W BYTOMIU UL PARKOWA 2 41902
R ADA MIEJSKA W BYTOMIU UL PARKOWA 2 41902 FAONETHERLANDS INTERNATIONAL CONFERENCE WATER FOR FOOD AND ECOSYSTEMS MAKE
FAONETHERLANDS INTERNATIONAL CONFERENCE WATER FOR FOOD AND ECOSYSTEMS MAKE