BIOE 11601161 SENIOR DESIGN 20042005 INITIAL HAZARD ANALYSIS –
Bioe 11601161 Senior Design 20042005 Initial Hazard Analysis –
Initial Hazard Analysis – Active Mobile Asthma Inhaler
BioE 1160/1161 Senior Design 2004-2005
Initial Hazard Analysis – Active Mobile Asthma Inhaler
Design Team:
Annemarie K. Alderson
Annie Saha
Stephanie T. Shaulis
Robert J. Toth
Advisor: Timothy E. Corcoran, PhD
Revision Date – November 17, 2004
This document presents a preliminary treatment of the predominant hazards associated with the proposed inhaler device being developed. The causes and effects of the described hazardous conditions, a qualitative assignment of general risk associated with the potential hazardous conditions, and the intended response of the system to the hazardous conditions are presented.
The most serious hazard associated with the inhaler device is the potential for a propellant mechanism malfunction. A malfunction of this particular mechanism can result in two major outcomes regarding the discharge of the medication; a complete misfire/inadequate ejection of the proper dosage or an exceedingly forceful ejection. This type of failure would be attributable to causes ranging from poor propellant mechanism design to manufacturing error to nonstandard use of the device. It should be noted that the design specifications for this device incorporate inherent safety regarding proper use of the device, i.e. if it is utilized according to the instructions in a manner consistent with its indications then propellant mechanism failure cannot be caused by patient use. However, if the device is utilized in a manner inconsistent with its intended use, malfunction of the propellant mechanism can be induced. The consequences of a propellant failure could be inadequate administering of the proper dosage of drug and the subsequent physiological effects of an untreated asthmatic episode. In addition, a chemical propelled ejection with a force in excess of specifications can potentially cause damage to the mouth, throat, and other components of the upper to mid respiratory tract. In general, the risk associated with this hazard is intermediate. The potential adverse consequences can be serious, however to probability of such a failure given adequate design and manufacturing controls is minimal. The system – consisting of the inhaler device – should respond to a propellant mechanism failure by simply becoming nonfunctional, which minimized the hazardous condition and whereby it can be mediated by replacement of the defective device with a new inhaler. This is not a serious problem considering that the device is intended to be single-use-only and disposable.
An additional hazard associated with the device is improper dosage loading. Since the device is designed to be single-use-only, the potential for inadequate or excessive dosage exists as an inherent limitation. The hazard is associated with the physiological effects of under- or over-medicating the asthmatic condition. In general, the risk associated with this hazard is minimal because, the device could be produced with varying dosages and the proper dosage device selected for the specific circumstances.
Since the device is designed to be completely sealed and hence water-proof, weather-proof, and reliable; the possibility exists for hazards associated with material compromise and subsequent premature drug dispensing. Again, the implied hazards involve lack of treatment of the condition. However, in addition, a compromise of the outer casing material (the development of a crack/tear, puncture, rupture, etc.) could create sharp plastic edges which pose a laceration hazard, or an unexpected ejection of the chemical propellant while; for example, the device was close to the face and eyes, which poses a reaction hazard. Such a hazard could be caused by a deficiency in the plastic casing material, an improper design choice of material, or an external device compromise such as inadvertent puncture. As indicated in prior examples, proper design and manufacturing controls and care with indicated usage would make the risk associated with this hazard minimal.
Finally, in terms of predominant hazards associated with the inhaler device being developed, a number of potential ergonomic/human factors hazards exist within the scope of proper utilization of the device. Since the device is designed to be able to be utilized in an active setting such as during physical activity like running or swimming, there are hazards involving complications to use during such activities. For example, the possibility exists for an impact between the device and the user during both active use and during the storage of the device at its peripheral location during activity. While properly administering medication from the device while running, the user’s attention may be diverted and an impact between the user and an unsuspecting third party may occur. This impact may subsequently cause a secondary collision of the device to the oral area of the user’s face, which could cause resulting damage. Also, for example, while being worn in a necklace fashion during running activity, the device may become caught on an article of clothing of the user and result in an injury to the user’s neck. These types of hazards are associated with proper use of the device and are attributable to the awareness of the user. Consequently, the risk associated with the device is low, and can be avoided by common awareness of the user’s environment.
This document was intended to serve as an initial treatment of the potential hazards associated with the proposed inhaler device being developed. It should be noted that additional hazards do exist, however a complete treatment of all potential hazards would be an encumbering exercise and that the predominant hazards discussed here represent the most serious and therefore those of most importance.
Tags: hazard, design, senior, initial, 11601161, analysis, 20042005
- PROYECTO DE NIMF MAYO DE 2004 PARA CONSULTA DE
- AGENDA PREŞEDINTELUI CONSILIULUI JUDEŢEAN MUREŞ PERIOADA 1 – 31
- KÁPOLNÁSNYÉKI KÖZÖS ÖNKORMÁNYZATI HIVATAL 2475 KÁPOLNÁSNYÉK FŐ U
- RYHMÄ 149 KILPAILUKÄYTÄNTEET 112019 LÄHTIEN UINTI JA UIMAHYPYT JOKA
- 1 MUNKABÉREK ILLETMÉNYEK (MINIMÁLBÉR GARANTÁLT BÉRMINIMUM) 3372010 (XII 27)
- CURRICULUM VITAE OCTOBER 2009 ROBERT J LEVINE MD SENIOR
- CHAPTER 1 INTRODUCTION IN EXERCISE OF POWERS CONFERRED
- ÅRSMÖTE NÄR ONSDAGEN DEN 21 MARS VAR IDROTTENS
- ROSKILDE LÆRERFORENING MØLLEHUSVEJ 8 – 4000 ROSKILDE ROSKILDE DEN
- LA LUZ TEST NOMBRE FECHA APELLIDOS CURSO 1
- ANEXO 5A REPORTE DE LA PRUEBA DE APROBACIÓN DE
- NAME DATE PERIOD GROUP MEMBERS MYSTERIOUS MINERALS
- CURRICULUM VITAE PROFESSOR HAKAM FAEQ ALHADIDI PERSONAL INFORMATION NAME
- APPUNTI DI BIOLOGIA LA DIVISIONE CELLULARE PROFSSA PATRIZIA MOSCATELLI
- Living Labs and Coproduction University Campuses as Platforms for
- MERCOSURCMCDEC N° 1217 MEMORANDUM DE ENTENDIMIENTO DE COOPERACION INTERNACIONAL
- SABTU 20 FEBRUARI 2010 PUKUL 102300 BELAJAR DARI BELANDA
- CURRICULUM VITAE CAIROUNIVERSITY FACULTY OF PHARMACY NAME NESRINE S
- PLATEFORME « POUR UN DROIT AU LOGEMENT OPPOSABLE »
- NRO 1765 ROSARIO 18 DE JULIO DE 2014 Y
- ROZBUDOWA I PRZEBUDOWA SZPITALA BIELAŃSKEIGO W WARSZAWIE PROJEKT
- 20 FAMOUS SCIENTISTS S PRIMARY 7 ROOM 16
- CURRICULUM VITAE 1 NAME SMANIKANDAN 2 GENDER MALE 3
- NADJA KOSSINSKAJA NADJA KOSSINSKAJA – A NATIVE OF
- RUTÓMETRO DE LA PRUEBA XX SUBIDA AL CASTILLOXIV MEMORIAL
- CURRICULUM VITAE SHUNFEN TZENG PHD PROFESSOR DEPARTMENT OF LIFE
- SMALL FATHER’S DAY TIE AND BOWTIE TEMPLATE LARGE FATHER’S
- SKRIFTLIG FORESPØRGSEL E468508 AF CHRISTEL SCHALDEMOSE (PSE) TIL KOMMISSIONEN
- AB BANKAS „FINASTA“ TEIKIA GALIMYBĘ JAUNIEMS ŽMONĖMS TOBULĖTI IR
- FORMULÁŘ K PŘÍJMU DO PORODNICE OTEC DÍTĚTE JMÉNO A
98ORD179 PAGE 8 NOT TO BE PUBLISHED 98ORD179 NOVEMBER
LIST OF JOURNALS FOR EDUCATORS AND SUBMISSION GUIDELINES ACADEME
WESTERN MILITARY INTERVENTION IN SYRIA? PURPOSE THIS IS AN
 SVEUČILIŠTE SJEVER ERASMUS+ PROGRAM KA1 INDIVIDUALNA MOBILNOST NASTAVNOG
SVEUČILIŠTE SJEVER ERASMUS+ PROGRAM KA1 INDIVIDUALNA MOBILNOST NASTAVNOGPOROZUMIENIE NR ……202… NA KORZYSTANIE Z SAMOOBSŁUGOWEGO SYSTEMU ODBIORU
APRUEBA LICITACION PUBLICA Nº 5505 ADQUISICION DE RETROEXCAVADORAS HIDRAULICAS
 PROGRAMA DE APOYO ESTRATÉGICO A PYMES 1 OBJETIVOS
PROGRAMA DE APOYO ESTRATÉGICO A PYMES 1 OBJETIVOS  BOP DE ALMERÍA NUMĂRUL 105 MARŢI 3 IUNIE
BOP DE ALMERÍA NUMĂRUL 105 MARŢI 3 IUNIE4 INFORMACIJA APIE VĖŽAIČIŲ LOPŠELĮDARŽELĮ 2010–2011 M M 4
INFORMACIJA APIE SUDARYTAS SUTARTIS PASLAUGŲ PIRKIMAI I PERKANČIOJI ORGANIZACIJA
SOSNOWIEC DNIA ………………… …………………………… IMIĘ I NAZWISKO …………………………… ROK
 COMUNICADO DE PRENSA NO 076 FECHA 02042009 IMPLEMENTARÁ CAPUFE
COMUNICADO DE PRENSA NO 076 FECHA 02042009 IMPLEMENTARÁ CAPUFE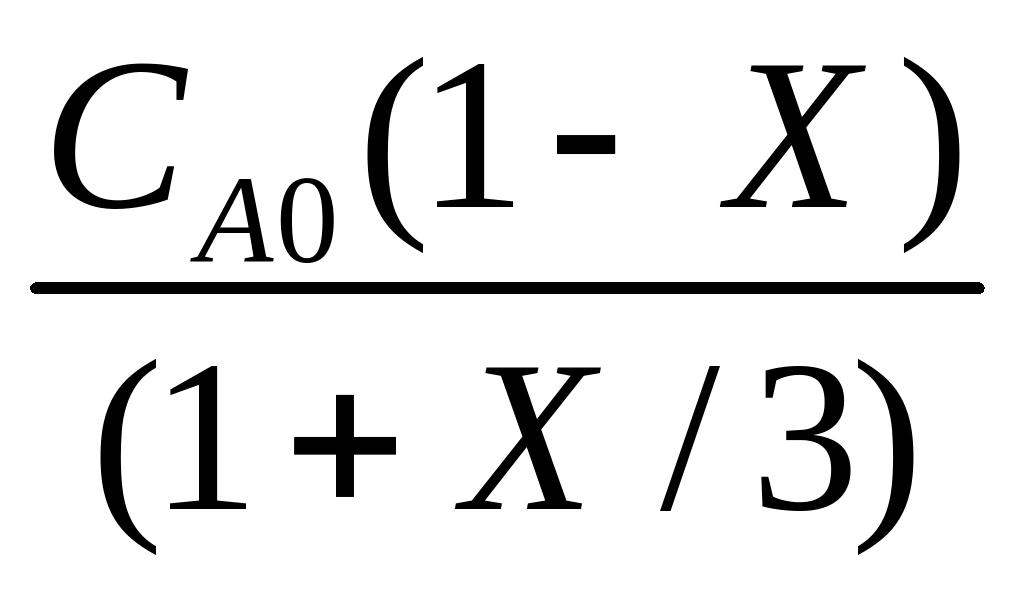 CHE 304 (SPRING 2007) LAST NAME FIRST QUIZ
CHE 304 (SPRING 2007) LAST NAME FIRST QUIZAAMFTS COMMISSION ON ACCREDITATION FOR MARRIAGE AND FAMILY THERAPY
8 PRIMERA REUNIÓN DEL CONSEJO ANDINO
 CHANGE REQUEST PROJECT NAME CHANGE NUMBER REQUESTED BY
CHANGE REQUEST PROJECT NAME CHANGE NUMBER REQUESTED BYDNIA 200 R PEŁNOMOCNICTWO DZIAŁAJĄC NA PODSTAWIE
NA TEMELJU ČLANKA 44 ZAKONA O LOKALNOJ I PODRUČNOJ
CURRICULUM VITAE JAMES B HICKS PAGE 14 CURRICULUM VITAE
ŠTUDIJNÝ ODBOR 11 HYGIENA CHOVU ZVIERAT A ŽIVOTNÉ