CLINICAL SCIENCE RESEARCH & DEVELOPMENT SERVICE GUIDANCE FOR MERIT
CLINICALLY RELEVANT ANATOMY 123 ULNAR NERVE ENTRAPMENTLONG ISLAND BHM CONCURRENT CLINICAL PLEASE COMPLETE
PSYCHOLOGY AND CLINICAL LANGUAGE SCIENCES UNIVERSITY OF READING
(INSERT AGENCY NAME) REPRODUCTIVE HEALTH PROGRAM CLINICAL POLICIES AND
006-17%20Clinical%20Psychiatrist%20%20Board%20%20037869
1 COURSE TITLE CLINICAL PRACTICUM IN AUDIOLOGY 2 2
Guidance for Merit Review Clinical Trials: Letter of Intent (LOI)
Clinical Science Research & Development Service
Guidance for Merit Review Clinical Trials: Letter of Intent (LOI)
I. Introduction
The application process for a CSR&D Merit Review Clinical Trial begins with a Letter of Intent (LOI). A full proposal may not be submitted without an approved LOI. An LOI provides CSR&D the opportunity to determine if a proposed trial will address a critically important and unique clinical question aimed at improving the healthcare of Veterans. It also allows CSR&D to provide feedback regarding the trial question, design, feasibility of enrolling the proposed population, etc. In some cases, CSR&D may suggest that an applicant utilize a non-trial RFA, or clarify that the proposed project falls within the purview of a research service other than CSR&D (e.g., Health Services Research and Development).
Clinical trial applications typically fall within the purview of the Clinical Trials Merit Review Panel (CLIN). CLIN reviews single-site or small multi-site clinical trials involving subject randomization and appropriate controls designed to assess potential effects of therapeutic intervention on intermediate physiological measures or studies aimed at definitive clinical outcomes.
In CSR&D, a clinical trial application that requires an approved LOI typically involves:
Human subject randomization and appropriate controls designed to assess the potential safety and/or effectiveness of an intervention, measured via a clinical outcome directly or indirectly with a surrogate endpoint such as a biomarker assessment
The utilization of interventions including, but not limited to, pharmaceutical compounds, agents, treatments or devices
Examples of clinical studies not requiring an approved LOI include:
Proposals designed to show equivalence between new and existing diagnostic devices or methods are usually not considered clinical trials for the purposes of this LOI.
Studies that focus on mechanisms underlying an intervention or those that evaluate tests and/or procedures aimed at diagnosing diseases (i.e., mechanistic and diagnostic studies) and that do not have a primary clinical outcome measure of interest do not require an LOI unless safety is of a major concern.
Observational, retrospective, and some prospective studies (e.g., a prospective study that has weight gain as the outcome and quantifies lifestyle habits as a risk factor) would not require an LOI.
Note: The definition of a CSR&D trial requiring an LOI may be less broad than that describing the requirements of a trial to be registered on clinicaltrials.gov: http://www.research.va.gov/resources/ORD_Admin/clinical_trials/. If you are uncertain as to whether or not an LOI is needed for your study, please send an e-mail to [email protected].
II. LOI Approval Considerations
Focus: A clinical trial submitted to the CSR&D Merit Review funding mechanism must be the sole objective of the application. This requires that pre-trial data collection or instrument development, etc., should be accomplished in prior studies supported separately (e.g., a clinical trial cannot be proposed as one specific aim of an application with multiple aims).
Waiver for Early Phase Trials: In rare circumstances, the Director, CSR&D, may approve an LOI for early phase trials (e.g., studies examining first use in humans, safety, dosing), provided safety data is available in at least two animal species. Early phase (Phase 0 and Phase 1) studies propose particular challenges that Phase 2-4 trials do not present in terms subject safety. Sufficient safety data should be presented as part of the LOI in order to justify approval as these studies are high risk projects where there is an unknown propensity for research-related injuries. Investigators proposing such studies must demonstrate that they and their study teams are experts in the particular subject area. Non-Veterans may not be proposed to be enrolled in early phase trials. This waiver request should be described in a memorandum, addressed to the Director, CSR&D.
Waiver for Enrolling Non-Veterans: Enrollment in CSR&D trials is limited to Veterans, unless a waiver to enroll non-Veterans is approved by Director, CSR&D. The request for a non-Veteran enrollment waiver should be submitted as part of the LOI approval process. This waiver request should be described in a memorandum, addressed to the Director, CSR&D, and should provide sufficient justification for the enrollment of non-Veterans and still support relevance of the trial to the Veteran population.
Eligibility: The Merit Review Award Program is an intramural funding mechanism to support investigator-initiated research conducted by eligible VA investigators at VA medical centers or VA-approved sites. CSR&D eligibility requirements will apply to all clinical trial submissions, but will not be evaluated in the LOI process described here. Please see the following for instructions regarding eligibility: http://www.research.va.gov/services/csrd/merit_review.cfm
III. LOI Submission Instructions
CSR&D will review submitted LOIs at any time; however, an LOI must be submitted at least 90 days in advance of the intended Merit Review submission deadline.
LOIs must be prepared using only letter-quality print, and all text must be prepared with at least 11-point font, with no more than 15 characters per inch and no more than 6 lines per inch. Page margins must be a minimum of 1 inch at each edge. Each LOI must contain the following materials:
A. VHA Research & Development Letter of Intent Cover Page (VA Form 10-1313-13) (http://www.research.va.gov/funding/process/forms.cfm )
B. LOI Text – Limited to three (3) pages. Please clearly and succinctly address each of the following in the specified order:
Proposed number of subjects = _____
Recruitment rate: subjects per month per site = _____
Duration = _____ years
Number of sites = _____
Total study budget = $__________
Describe the category of study using FDA Phase Definitions (e.g., Phase 0, 1, 2, 3 or 4; http://clinicaltrials.gov/ct2/help/glossary/phase)
Objectives of the proposed clinical trial and hypothesis to be tested
Description of the primary endpoint
Description of proposed intervention and control/s
Scientific Rationale
Background Data/Preliminary Studies conducted to support application. If Phase 1, evidence for safe use in at least two animal species must be provided in a waiver request attached to this LOI.
Study Design/Methods
Sample size determination and description of subject population.
Please state if you will propose the enrollment of non-Veterans or will enroll only Veterans. If enrolling non-Veterans, estimate the ratio of Veterans to non-Veterans that will be enrolled in this study. If proposing to enroll non-Veterans, a separate waiver request must be attached to this LOI; please refer to section II (B).
Data Analysis Plan and Description of Biostatistical Expertise
Describe any problems or challenges anticipated regarding timely start up and execution (including whether the study will be FDA regulated, agreements with industry or other non-VA entity, patents/licensing, etc).
Are there any competing trials locally or nationally on this topic in this subject population? If so, describe and indicate distinction; if none, indicate NONE. See www.clinicaltrials.gov as one source for this information.
How would results impact clinical practice and/or plans for further studies?
C. Supplemental Pages:
PI’s Biographical Sketch. Attach completed form listed under “Additional Format Pages”, Biographical Sketch Format Page: http://vaww.research.va.gov/funding/electronic-submission.cfm Please highlight specific clinical trials experience.
D. Addenda (If necessary)
Waiver request for enrollment of non-Veteran subjects; please refer to Section II (B). This waiver must be fully justified and submitted with the LOI in a memorandum format signed by ACOS for Research. This waiver will be evaluated for risks and justification related to feasibility and generalizability to the Veteran population.
Waiver request to approve an early phase trial; please refer to Section II (B) e.g., first use in humans/safety/dosing. This waiver must be fully justified in a memorandum signed by ACOS for Research. The waiver will be evaluated for evidence of pre-clinical data as well as potential risks to Veteran participants, and clinical implementation.
IV. Submission of an LOI
LOIs may be e-mailed to [email protected] from the local VA facility research office. Questions relating to the LOI preparation and submission process may also be directed to [email protected].
The deadline to submit a clinical trial LOI is at least 90 days prior to the scheduled Merit Review application deadline. December 1 is the deadline for the Spring review cycle and June 1 is the deadline for the Fall review cycle. If the deadline falls on a weekend, the due date is the next business day.
Applicants are strongly encouraged to submit LOIs as early as possible.
V. Approval
If an LOI for a clinical trial is approved, the approval letter must be included in the proposal application submission (Letters section). A submitted clinical trial proposal must not deviate in specific aims from the original approved clinical trial LOI. CSR&D may administratively withdraw from review any proposal that substantially deviates from what was described and approved at the LOI stage.
An approved clinical trial LOI is valid for proposal submission for the next three consecutive Merit submission cycles. Once an application is submitted, Standard Merit Review limitations apply; that is, each proposal may undergo an initial review and, if necessary, two resubmissions (3 strikes).
Note: Proposals submitted in response to the Clinical Trials RFA must include a copy of the LOI approval and the approved waivers (e.g., non-Veteran enrollment waiver) as applicable. Failure to do so may result in the proposal being administratively withdrawn from review.
E-mail questions to: [email protected].
Effective: May 01, 2014
CSR&D
LOI for Clinical Trials Page
1 NEONATAL RESPIRATORY DISTRESS INCLUDING CPAP CLINICAL LEARNING RESOURCE
1066 DEMENTIA RESEARCH GROUP PILOT STUDIES INDEPENDENT CLINICAL ASSESSMENT
10AP17] CLINICAL AND ENDOSCOPIC COMPARISON OF THE SINGLE USE
Tags: clinical science, for clinical, clinical, science, guidance, development, research, merit, service
- Informacja DLA Kandydatów NA Studia II Stopnia na Wydziale
- BRIDGEND COUNTY BOROUGH COUNCIL TOWN POLICE CLAUSES ACT 1847
- SOCIAL CAPITAL ASSESSMENT TOOL ANIRUDH KRISHNA AND ELIZABETH
- POVZETEK REVIZIJSKEGA POROČILA O PRAVILNOSTI POSLOVANJA AGENCIJE REPUBLIKE SLOVENIJE
- ALL OF THE INFORMATION IN THIS DOCUMENT IS THE
- mu Alpha Theta National Convention Hawaii 2005 Solutions –
- CONTRACT DE ACHIZITIE PUBLICA DE PRODUSE NR DATA
- UNIVERSIDAD METROPOLITANA FACULTAD DE CIENCIAS ADMINISTRATIVAS EJERCICIOS INTERES
- ECEC DAILY SCHEDULE MAY VARY FROM DAY TO DAY…
- LA MAGIA DE LA LUNA DE JOSÉ ANTONIO MARTÍNEZ
- NA OSNOVU ČLANA 70 STAV 1 TAČKA 2 USTAVA
- BANAL NA KORDERO NG DIYOS KANYANG BANAL NA PILOSOPIYA
- “EMPLEO CON APOYOGABINETES DE ORIENTACIÓN E INSERCIÓN LABORAL” (MODELO
- TG2571 FLOX 20090401 7 S TG2571 ORIGINAL
- A D EMANDE DE LOGEMENT À LOYER MODIQUE (LES
- AGENDA ELECTRÓNICA ESPECIFICACIÓN DE REQUISITOS LA FINALIDAD DE
- 04 NISAN 2007 SAYI 26483 GELİR VERGİSİ KANUNU VE
- „UPARTE ” DZIECI NEGATYWIZM DZIECIĘCY „BO NAWET MIŁUJĄC KRZYWDZĘ
- PLAN GENERAL DE SEVILLA MESA 4 LA SEVILLA SOLIDARIA
- LETTER OF UNDERTAKING DATE TO
- IGUAL DIFERENTE RODEA Y PINTA LOS IGUALES ¿CUÁNTOS HAY?
- AULA DE AUDICIÓN Y LENGUAJE CURSO HOJA DE REGISTRO
- LOS JÓVENES NAVARROS VALORAN POSITIVAMENTE EL SISTEMA DEMOCRÁTICO AUNQUE
- A DECISION SUPPORT TOOL FOR SOCIALLY RESPONSIBLE CONSCIOUS INVESTORS
- 4 HƯỚNG DẪN CÁCH GHI NGÀNH NGHỀ TRONG HỒ
- POWERPLUSWATERMARKOBJECT16040720 SAP CONCUR RELEASE NOTES CONCUR REQUEST STANDARD EDITION
- APPLICATION FOR A NORTH CAROLINA LICENSE TYPE OR PRINT
- OUTLINE I GENERAL CONSIDERATIONS A RISK FACTORS 1 POSITIVE
- ENCUESTA INICIAL HAVE YOU GOT A COMPUTER AT HOME?
- ZAKRES KOMPETENCJI DYREKTORA PUP REALIZACJA ZADAŃ OKREŚLONYCH W
ISPS GIVEN PROVISIONAL PERMISSION TO OFFER VPN SERVICES STATUS
УТВЕРЖДАЮ ЗАМЕСТИТЕЛЬ ПРЕДСЕДАТЕЛЯ ДЕГУСТАЦИОННОЙ КОМИССИИ НАЧАЛЬНИК УПРАВЛЕНИЯ ПИЩЕВОЙ И
 ENCOURAGING EFFECTIVE DIALOGUE AND ADVOCACY IN NIGERIA THE BETTER
ENCOURAGING EFFECTIVE DIALOGUE AND ADVOCACY IN NIGERIA THE BETTERESCRIVIM DIEM EN VALENCIÀ PER LAURA GARCIA LA
 PROGRAM TEDNA BOJA PROTI RAKU OD 4 3
PROGRAM TEDNA BOJA PROTI RAKU OD 4 3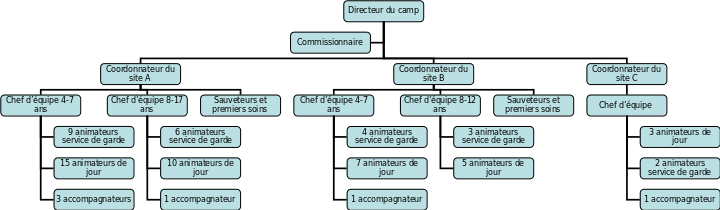 ASSOCIATION DES CAMPS DU QUÉBEC GUIDE DES BONNES PRATIQUES
ASSOCIATION DES CAMPS DU QUÉBEC GUIDE DES BONNES PRATIQUESNATIONAL INDIAN GAMING COMMISSION MICS AUDIT CHECKLIST CAGE (CG)
GRAD SUBOTICA MESNA ZAJEDNICA „PROZIVKA“ SAVET MESNE ZAJEDNICE BROJ90122014
DE LA VISIÓN Y ENIGMA CUANDO SE CORRIÓ ENTRE
HEADS OF TERMS BETWEEN [COMMUNITY GROUP] AND LANDOWNER [PROPERTY]
 Z USATZINFORMATIONEN ZUR KATASTROPHENSCHUTZÜBUNG ALLGEMEINE INFOS ZUR ÜBUNG DIE
Z USATZINFORMATIONEN ZUR KATASTROPHENSCHUTZÜBUNG ALLGEMEINE INFOS ZUR ÜBUNG DIEDECRETO FORAL 1012000 DE 27 DE DICIEMBRE POR EL
 M ONTI A 2004 AMBIENTE NATURAL I TERCER MÓDULO
M ONTI A 2004 AMBIENTE NATURAL I TERCER MÓDULOJAVALANGNULLPOINTEREXCEPTION AT COMUQIANSOFTDOCUMENTSERVERSERVICEDOWNLOADFILE(SERVICEJAVA403) AT SUNREFLECTGENERATEDMETHODACCESSOR41INVOKE(UNKNOWN SOURCE) AT SUNREFLECTDELEGATINGMETHODACCESSORIMPLINVOKE(DELEGATINGMETHODACCESSORIMPLJAVA25) AT
8 ORDENANZA Nº 114412010 EXPTENº 38492010–HCD VISTO LA AUSENCIA
 V PREMIOS A LA MEJOR PRÁCTICA EN MOVILIDAD SOSTENIBLE
V PREMIOS A LA MEJOR PRÁCTICA EN MOVILIDAD SOSTENIBLE RESOLUCIÓN NO DE HOJA NO 99
RESOLUCIÓN NO DE HOJA NO 99  VORNAME NACHNAME DATUM DER VERANSTALTUNG TITEL DER VERANSTALTUNG TANDEMPARTNERIN
VORNAME NACHNAME DATUM DER VERANSTALTUNG TITEL DER VERANSTALTUNG TANDEMPARTNERIN TERSANELER CAD NO26 34944 TUZLAİSTANBUL PHONE +90 216 581
TERSANELER CAD NO26 34944 TUZLAİSTANBUL PHONE +90 216 581 STATEMENT COMITÉ TRABAJADORES MIGRATORIOS 15TH SESSION (1223 SEPTEMBER 2011)
STATEMENT COMITÉ TRABAJADORES MIGRATORIOS 15TH SESSION (1223 SEPTEMBER 2011)