SALMONELLOSIS ACTIVITIES IN 2009 SALMONELLOSIS DR C POPPE PUBLIC
NEW YORK STATE CATTLE HEALTH ASSURANCE PROGRAM SALMONELLOSIS MODULESALMONELLA (NONTYPHOIDAL) SALMONELLA SPP ARE BACTERIA THAT CAUSE SALMONELLOSIS
SALMONELLOSIS ACTIVITIES IN 2009 SALMONELLOSIS DR C POPPE PUBLIC
SALMONELLOSIS IS AN IMPORTANT DISEASE FOR BOTH ANIMALS AND
Disease name
Salmonellosis
Activities in 2009
Salmonellosis
Dr C. Poppe
Public Health Agency of Canada, Laboratory for Foodborne Zoonoses
110 Stone Road West, Guelph, Ontario N1G 3W4 Canada
Tel.: (1-519) 822-3300, Fax: (1-519) 822-2280
E-mail: [email protected]
Summary of general activities related to the disease
1. Serotyping and phagetyping of Salmonella cultures for diagnostic, research and surveillance purposes
The OIÉ Reference Laboratory for Salmonellosis is a national Salmonella surveillance centre, contributing laboratory support, Salmonella cultures and submissions data to the national enteric pathogen surveillance system of the Public Health Agency of Canada, the Canadian Integrated Program for Antimicrobial Resistance (http://www.phac-aspc.gc.ca/cipars-picra/index.html), and PulseNet Canada (the Canadian listserve for PFGE/molecular methods, bacterial outbreak investigations and surveillance). The Laboratory also supports provincial government Salmonella monitoring programs and research programs of government institutions and universities in Canada and internationally.
A total of 5,169 Salmonella cultures isolated from animals and their environment, foods, feeds and other sources in Canada during the year 2009 were identified serologically using the antigenic formulae of Grimont and Weill (2007). They belonged to 196 serovars. The commonest serovars were S. Kentucky (854 cultures), S. Heidelberg (800), S. Enteritidis (784) and S. Typhimurium (492 cultures, consisting of 250 S. Typhimurium var. Copenhagen and 242 S. Typhimurium). Other commonly isolated serovars were S. Schwarzengrund (193), S. Mbandaka (156), S. Hadar (143), S. Infantis (129) and S. Senftenberg (113). Three hundred and twenty one Salmonella cultures belonging to 127 serovars were identified for internal and external quality control assurance purposes. Among the animal source submissions, most Salmonella isolates were from chickens (2,290 cultures), pigs (475), turkeys (290), cattle (169) and horses (24). The most common food isolates were from chicken (562) and the most common isolates from environmental sources were from litter and manure (146). Also, 199 isolates from feed and feed ingredients were serotyped. Salmonella Kentucky, S. Enteritidis and S. Heidelberg were the most frequently isolated serovars from chicken (588, 525 and 446 cultures, respectively). Salmonella Typhimurium with S. Typhimurium var. Copenhagen, and S. Derby were the most frequently isolated serovars from pigs (126 and 70 cultures, respectively) and S. Schwarzengrund from turkeys (115 cultures). Salmonella Typhimurium and S. Typhimurium var. Copenhagen were the most frequent serovars from cattle (25 and 59 cultures, respectively). The most frequently isolated serovars from chicken as a food source were Salmonella Heidelberg (163), S. Kentucky (149) and S. Enteritidis (91 cultures). Salmonella were isolated from a wide variety of species such as chickens, pigs, turkeys, other avian species, cattle, horses, ducks, reptiles, cats, dogs, geese, sheep, mink, rodents and other animal species, from environmental samples such as litter and manure, from equipment and buildings, from dust, from water and soil samples, from foods (chicken, pork, beef and turkey), from other food sources such as fruits, vegetables, fish, herbs and spices, and from feed, feed ingredients and fertilizer.
Phagetyping of 793 S. Heidelberg isolates with the typing scheme of Demczuk et al. (J. Clin. Microbiol. 2003; 41: 4279-4284) showed that phage type (PT) 19 was the commonest PT (286 cultures). A total of 487 S. Typhimurium isolates, including S. Typhimurium var. Copenhagen were characterized by the phage typing scheme of Anderson et al. (J. Hyg. 1977; 78: 297-300); phagetype 104 was the commonest PT identified (103 cultures) followed by PT8 (55 cultures). Typing of 783 S. Enteritidis strains with the scheme of Ward et al. (Epidemiol. Infect. 1987; 99: 291-294) showed that most belonged to PT8 (357 cultures), PT51 (113) and PT13a (94 cultures); none of the cultures typed as PT4.
2. Production and distribution of diagnostic reagents
Cultures are available from the Salmonella strain collection for research and diagnostic purposes.
Activities
specifically related to the mandate
of OIE Reference
Laboratories
3. International harmonisation and standardisation of methods for diagnostic testing or the production and testing of vaccines
The OIÉ Reference Laboratory for Salmonellosis has been accredited by the Standards Council of Canada since 1997 and its diagnostic procedures comply with the requirements of the ISO/IEC Guide 17025. The Laboratory has been a member of the WHO Global Salmonella Surveillance network ([email protected]) since 2000. The Laboratory is listed on the Global Salm-Surv web page (http://www.who.int/salmsurv/en). The Laboratory has successfully participated in a yearly External Quality Assurance System (EQAS) for Salmonella serotyping among Global Salm-Surv member laboratories since 2001.
4. Preparation and supply of international reference standards for diagnostic tests or vaccines
Salmonella antisera, Salmonella cultures and other diagnostic supplies were provided to researchers and diagnosticians in Member Countries. The Reference Laboratory submitted a new serovar to the WHO Collaborating Center for Reference and Research on Salmonella at l’Institut Pasteur in Paris, France. The isolate was confirmed having the 51:y:1,2 antigenic formula and was named Salmonella enterica subsp. enterica Rosslyn. It had been isolated from a ball python snake from Rosslyn, Alberta, Canada in 2008.
5. Research and development of new procedures for diagnosis and control
The following topics are being studied: The epidemiology, pathogenesis and control of salmonellosis in animals and humans; antimicrobial resistance and trends in resistance of Salmonella; the putative association between antimicrobial resistance and virulence in Salmonella; further characterization of Salmonella by serotyping, phagetyping, PFGE, plasmid profiles, and by PCR to identify antimicrobial resistance, virulence and other genes; sequencing of DNA and other molecular typing and research techniques; alternative molecular methods to traditional serotyping.
6. Collection, analysis and dissemination of epizootiological data relevant to international disease control
Comprehensive monthly and annual reports were composed and sent to national and international organisations.
7. Provision of consultant expertise to OIE or to OIE Members
The OIÉ Expert on Salmonellosis at our Laboratory reviewed the Report of the Meeting of the OIE AD HOC Group on Salmonellosis, held in Paris, 4-6 August 2009. The Expert also reviewed and commented on Chapter 6.4 titled “Biosecurity Procedures in Poultry Production” and Chapter 6.5 on “Prevention, detection and control of Salmonella in poultry” (OIÉ Terrestrial Animal Health Standards Commission/September 2009 documents).
8. Provision of scientific and technical training to personnel from other OIE Members
Nil.
9. Provision of diagnostic testing facilities to other OIE Members
The Salmonella Reference Laboratory examined 49 ovine putative Salmonella isolates that originated from the College of Agriculture and Veterinary Medicine, Jimma University, Ethiopia.
10. Organisation of international scientific meetings on behalf of OIE or other international bodies
Nil.
11. Participation in international scientific collaborative studies
A miniature Salmonella molecular typing array has been developed based on the ClonDiag ArrayStripTM (AS) system together with researchers at the National Microbiology Laboratory (NML) in Winnipeg, Canada and the Veterinary Laboratory Agencies (VLA) of the United Kingdom. The array is currently undergoing final optimization in preparation for a blind panel assessment which is planned to begin in March of 2010. If the results from the blind panel are successful then the array will be included in a large scale comparison of traditional Salmonella serotyping with new molecular typing techniques at the VLA Reference Laboratory. A large scale validation will then be completed prior to commercialization with Inverness Medical.
12. Publication and dissemination of information relevant to the work of OIE (including list of scientific publications, internet publishing activities, presentations at international conferences)
Presentations at international conferences and meetings
Weir E, Martin L, Boerlin P. Exposure to antimicrobials and virulence gene expression in Salmonella. 3rd ASM Conference on Salmonella: Biology, Pathogenesis and Prevention. Aix-en-Provence, France. October 5-9, 2009, Poster presentation.
Franklin K, Lingohr EJ, Bodrossy L, Anjum M, Villegas A, Clark CG, Kropinski AM. Development of a miniaturized tube array for molecular typing of Salmonella. 3rd ASM Conference on Salmonella: Biology, Pathogenesis and Prevention. Aix-en-Provence, France, October 5-9, 2009. Poster presentation.
Boerlin P. Le role des produits carnés dans la transmission de bactéries résistantes chez l’humain. Colloque Zoonoses et Santé Publique. Vingt-deuxièmes Entretiens Jacques Cartier, Lyon, France, November 30, 2009.
Scientific publications in peer-reviewed journals
Sibhat B, Zewde BM, Zerihun A, Muckle A, Cole L, Boerlin P, Wilkie E, Perets A, Mistry K, Gebreyes WA. Salmonella serovars and antimicrobial resistance profiles in beef cattle, slaughterhouse personnel and slaughterhouse environment in Ethiopia. Zoonoses Public Health 2009; Dec 23 [Epub ahead of print].
Murphy C, Reid-Smith RJ, Prescott JF, Bonnett BN, Poppe C, Boerlin P, Weese JS, Janecko N, McEwen SA. Occurrence of antimicrobial resistant bacteria in healthy dogs and cats presented to private veterinary hospitals in southern Ontario: A preliminary study. Can Vet J 2009; 50:1047-1053.
Kozak GK, Pearl DL, Parkman J, Reid-Smith RJ, Deckert A, Boerlin P. Distribution of sulfonamide resistance genes in Escherichia coli and Salmonella isolates from swine and chickens at abattoirs in Ontario and Québec, Canada. Appl Environ Microbiol 2009; 75:5999-6001.
Boerlin P, Reid-Smith RJ. Antimicrobial resistance: its emergence and transmission. Anim Health Res Rev 2008; 9:115-126.
Farzan A, Friendship RM, Cook A, Pollari F. Occurrence of Salmonella, Campylobacter, Yersinia enterocolitica, Escherichia coli O157 and Listeria monocytogenes in swine. Zoonoses Public Health 2009; Jul 23 [Epub ahead of print].
Hag Elsafi HE, Nor Elmadiena MM, El Hussein AA, Siddig MA, Muckle CA, Cole L, Wilkie E, Mistry K. Salmonella Umbadah: a new Salmonella serovar isolated from cattle in Sudan. Trop Anim Health Prod 2009; 41:1605-1606.
Clark CG, Kropinski AM, Parolis H, Grant CC, Trout-Yakel KM, Franklin K, Ng LK, Paramonov NA, Parolis LA, Rahn K, Tabor H. Escherichia coli O123 O antigen genes and polysaccharide structure are conserved in some Salmonella enterica serogroups. J Med Microbiol 2009; 58:884-894.
Villegas A, She YM, Kropinski AM, Lingohr EJ, Mazzocco A, Ojha S, Waddell TE, Ackermann HW, Moyles DM, Ahmed R, Johnson RP. The genome and proteome of a virulent Escherichia coli O157:H7 bacteriophage closely resembling Salmonella phage Felix O1. Virol J 2009; 6:41.
Other communications
None.
13. Inscription of diagnostic kits on the OIE Register
i) Did you participate in expert panels for the validation of candidate kits for inscription on the OIE Register?
No.
ii) Did you submit to the OIE candidate kits for inscription on the OIE Register?
No.
_______________
Annual
reports of OIE Reference Laboratories and Collaborating Centres,
2009
Tags: salmonellosis activities, on salmonellosis, salmonellosis, activities, poppe, public
- PLAN DZIAŁALNOŚCI ZAKŁADU UBEZPIECZEŃ SPOŁECZNYCH1) NA ROK 2019 DLA
- COMBINED MANUAL ISSUE DATE 062021 REQUIREMENTS FOR CAREGIVERS UNDER
- DOCUMENTO PRIVADO DE CONSTITUCION DE SOCIEDAD POR ACCIONES SIMPLIFICADA
- INF19 PAGE 5 INF19 ECONOMIC COMMISSION FOR EUROPE INLAND
- DATA EVALUATION REPORT ON THE ACUTE TOXICITY OF {TAI
- AUDIENCIAS PÚBLICAS DEL LUNES 17 AL VIERNES 21 DE
- URZĄD MIASTA I GMINY W KOLONOWSKIEM SO556152015 ZATWIERDZAM
- MERSEYSIDE & CHESHIRE PALLIATIVE CARE NETWORK AUDIT GROUP SUPRAREGIONAL
- 2 FORDULÓ 1 KÉT POZITÍV SZÁM ARÁNYA KÜLÖNBSÉGÜK
- PRACTICAL STRATEGIES FOR MANAGING CHILDRENADOLESCENTS WITH NEURODEVELOPMENTAL DISORDERS INCLUDING
- VYHLÁŠENÍ VÝBĚROVÉHO ŘÍZENÍ VÝBĚROVÉ ŘÍZENÍ NA REZIDENČNÍ MÍSTO
- SINFONÍA Nº 9 EN RE MAYOR OP 125 “CON
- 18 BRKALLIANZ – GERMAN CRPD ALLIANCE (EDS) ALLIANCE OF
- Cacfp Weekly Menu Template With Quantities for Children for
- GESTION DES DONNÉES MANQUANTES ABERRANTES ET INCOHÉRENTES DANS L’ÉTUDE
- KOMUNIKAČNÍ PLÁN RPS 2006 ÚVOD A RÁMCOVÁ STRATEGIE
- NAME PERIOD T EMPERATUREPRECIPITATION BIOME ACTIVITY PURPOSE
- MI A SZAKKOLLÉGIUM MIÉRT ÉRDEMES SZAKKOLLÉGISTÁNAK LENNI? A JELENLEG
- AUDIENCIAS DEL MES DE MARZO DE 2012 PRIMERA SALA
- MONITOROWANIE W ANESTEZJOLOGII I INTENSYWNEJ TERAPII INTENSYWNY NADZÓR
- SAMPLE RESIGNATION LETTER [YOUR NAME] [STREET • CITY •
- MORSE FALLS SCALE ASSESSMENT SUBMITTED BY BETH ALLER RN
- KRAJEVNA SKUPNOST KISOVEC LOKE ZAPISNIK 11 SEJE
- 45 PERATURAN MENTERI KESEHATAN REPUBLIK INDONESIA NOMOR TENTANG STANDAR
- ESPOL ICQA 1ERA EVALUACIÓN QUÍMICA GENERAL I
- UNIVERSIDAD DEL MAGDALENA ESPECIALIZACIÓN EN LOGÍSTICA Y TRANSPORTE INTERNACIONAL
- NOMBRE DE LA EMOCIÓN IRARABIA ACTIVIDADES SESIÓN 1º ¿QUÉ
- ZAŁĄCZNIK NR 1 SPECYFIKACJA STRONY INTERNETOWEJ OPIS PRZEDMIOTU ZAMÓWIENIA
- BRAZILIAN SOIL SCIENCE ACTIVITIES IN ANTARCTICA STARTED IN 2002
- TÉCNICO SUPERIOR UNIVERSITARIO EN ACUICULTURA ÁREA ADMINISTRACIÓN Y EVALUACIÓN
 Instituto Universitario de Electroquímica Institut Universitari D’electroquímica Lectures in
Instituto Universitario de Electroquímica Institut Universitari D’electroquímica Lectures inISIÖLÇER VE ISI SAYACI SÖKME TAKMA ELEMANI (SEVIYE 3)
PARKSIDE STUDENT EVALUATION RESULTS WHERE WILL I LIVE LESSONS
 OUR REFERENCE 30814 FOI 89961 APRIL 2014 FREEDOM OF
OUR REFERENCE 30814 FOI 89961 APRIL 2014 FREEDOM OFUDRUŽENJE VLASNIKA LOKALA ‚‚TPC KALČA‚‚ NIŠ UL OBRENOVIĆEVA BR
INSTRUCTIONS THIS STORY DEALS WITH THE RELEASE OF CLERY
ZAŁĄCZNIK NR 1 DO REGULAMINU POMOCY MATERIALNEJ DLA UCZESTNIKÓW
 INFORME FINAL ANÁLISIS PROSPECTIVO DE MECANISMOS FINANCIEROS PARA LA
INFORME FINAL ANÁLISIS PROSPECTIVO DE MECANISMOS FINANCIEROS PARA LA 20 SEPTEMBER 2005 G436 BIOGEOCHEMISTRY ASSOC PROF
20 SEPTEMBER 2005 G436 BIOGEOCHEMISTRY ASSOC PROF3 QUATRIÈME RÉUNION DES AUTORITÉS CHARGÉES DES OEASERKXXXIV POLITIQUES
419 STATUTTER FOR MOBO – FONDET 1 BAKGRUNN MOBOFONDET
 APPLICATIONS ARE TO BE RETURNED TO POLLUTION TEAM PUBLIC
APPLICATIONS ARE TO BE RETURNED TO POLLUTION TEAM PUBLICANEXO 1 ARTÍCULOS DEL CÓDIGO PENAL TEXTO ÚNICO CÓDIGO
ESTADO ACTUAL DE LA CUESTIÓN TÍTULO DAÑOS Y PERJUICIOS
FORMATO PLANIFICACIÓN CON ELEMENTOS DE LAS BASES CURRICULARES 2013
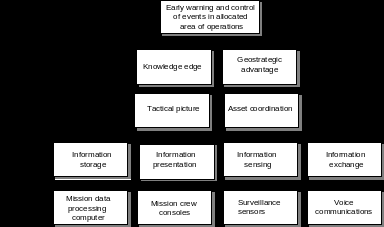 PROCEEDINGS OF THE FIFTH AUSTRALIAN AVIATION PSYCHOLOGY SYMPOSIUM NOVEMBER
PROCEEDINGS OF THE FIFTH AUSTRALIAN AVIATION PSYCHOLOGY SYMPOSIUM NOVEMBER22042015 TARIH VE 28095 SAYILI BAKANLIK OLURU ILE YÜRÜRLÜĞE
SUGERENCIA Nº 3 AL AVANCE DEL PLAN GENERAL DE
 PO BOX 626 VERSAILLES KY 40383 TELEPHONE 8598737300
PO BOX 626 VERSAILLES KY 40383 TELEPHONE 8598737300  FORDE ABBEY ANNUAL CHARITY SUMMER FAIR THURSDAY 25 JULY
FORDE ABBEY ANNUAL CHARITY SUMMER FAIR THURSDAY 25 JULY