MONROE COMMUNITY COLLEGE HUMAN SUBJECTS RESEARCH CONSENT FORM PAGE
1483 SNOWS MILL RD MONROE GA 30655 7702679017 PHONECITY OF MONROE STANDARD SPECIFICATIONS AND DETAIL MANUAL 0400
CITY OF MONROE STANDARD SPECIFICATIONS AND DETAIL MANUAL PREFACE
CITY OF MONROE STORMWATER ADMINISTRATIVE MANUAL 100107 ADMINISTRATIVE MANUAL
CITY OF MONROE YOUTH CODE OF CONDUCT PARTICIPANT’S NAME
CITY OF SYLVANIA UTILITIES OFFICE 6730 MONROE STREET SYLVANIA
SINCLAIR COMMUNITY COLLEGE
Monroe Community College
Human Subjects Research Consent Form
Page
MONROE COMMUNITY COLLEGE
Human Subjects Research
Consent to Participate in Research
Study Title:_____________________________________________________________
Researcher:_____________________________________________________________
This is a consent form for participation in a research study. It contains important information about this study and what to expect if you decide to participate.
Your participation is voluntary. You may refuse to participate in this study. If you decide to take part in the study you may leave the study at any time. If you are a student or employee at Monroe Community your decision will not affect your grades or employment status.
Please review the information carefully. Feel free to ask questions before making your decision whether or not to participate. If you decide to participate, you will be asked to sign this form and will receive a copy of the form.
Purpose: A statement that the study involves research, an explanation of the purpose(s) of the research, the number of participants anticipated to be enrolled in the study and the expected duration of the participant’s participation, a description of the procedures to be followed.
Conflict of Interest Statement: Investigator should state whether he/she is receiving payment for conducting this research.
Risks: A description of any reasonably foreseeable risks or discomforts to the participant.
Benefits: A description of any benefits to the participant or to others which may reasonably be expected from the research.
Confidentiality of Records: Statement about confidentiality of records
Treatments: For research involving more than minimal risk, an explanation as to whether any treatments are available if injury occurs, and, if so, what they consist of, or where further information may be obtained.
Contacts: An explanation of whom to contact for answers to pertinent questions about the research and research participant’s rights, and whom to contact in the event of a research-related injury to the participant.
Contact Persons
The consent form must address three (3) areas for participant’s questions namely, questions about the research itself, questions about research related injury and questions about the participant’s rights. Examples of acceptable wording for this section:
“For more information concerning this research you should contact (specify name) at (telephone number) (Note: this person is usually the principal investigator).”
“If you believe that you may have suffered a research related injury, contact (specify name) at (telephone number) who will give you further instructions.
“If you have any questions about your rights as a research participant, you may contact the IRB chairperson at Monroe Community College at (585)-292-2740 or e-mail [email protected]”.
Voluntary: A statement that participation is voluntary, that refusal to participate will involve no penalty to the participant and that the participant may discontinue participation at any time without penalty.
Termination Anticipated circumstances under which the participant’s participation may
Study: be terminated by the investigator without regard to the subject’s consent. A statement that the investigator, Monroe Community College or Monroe Community College IRB have the right to terminate the protocol.
Costs: Any additional costs to the participant that may result from participation in the research.
Signature Page Requirements
Monroe Community College’s Institutional Review Board requires that a consent form provide a place for the printed name and signature of the person obtaining consent, the participant, and a witness (if the research is greater than minimal risk).
The requirement of a witness is not required by federal regulations; however Monroe Community College’s Institutional Review Board requires this signature for all research with greater than minimal risk to study participants. The intent of the witness is to acknowledge that the participant is giving their consent freely and without reservation, the witness does not need to be present for the entire informed consent process. The witness must be an individual not directly involved in the conduct of the study.
Participant
I have read (or have had read to me) the contents of this consent form and have been encouraged to ask questions. I have received answers to my questions. I give my consent to participate in this study. I will receive a signed copy of this form for my records and future reference.
________________________ _____________________________ ________
Printed name of participant Signature of participant Date
Person Obtaining Consent
I have read this form to the participant and/or the participant has read this form. An explanation of the research was given and questions from the subject were solicited and answered to the participant’s satisfaction. In my judgment, the participant has demonstrated comprehension of the information.
_____________________ __________________ ________
Print Name Signature Date
Witness (if research greater than minimal risk)
The participant has indicated to me that the research has been explained to the participant, that the participant has read (or had read to the participant) this consent form, and that all of the participant’s questions have been answered. In my judgment, the participant is voluntarily signing this consent form.
_____________ _________ ________________________ ____________
Witness Name (Print) Signature Date
CONDADO DE MONROE FLORIDA SOLICITUD DE BOLETA DE
III THE UNIVERSITY OF LOUISIANA AT MONROE ONLINE DOCTORAL
MONROE COMMUNITY COLLEGE HUMAN SUBJECTS RESEARCH CONSENT FORM PAGE
Tags: college human, community college, subjects, research, community, consent, human, college, monroe
- 2324042020 R MUZYKA KL IVA – DANUTA KUSIAK TEMAT
- CURRICULUM VITAE TAMER M ESSAM EZELDEEN MOBILE
- CASE NUMBER DPP1281 CASE NAME REV 919 922 KAR
- PROYECTOS RESIDENCIAL PANORAMA VPO PRADO DEL
- PREDMET PORESKA I CARINSKA POLITIKA NAKNADA KAO IZVOR SREDSTAVA
- THE PERINATAL CENTER PLLC AUTORIZACION PARA LA DIVULGACION DE
- TO PORTOFOLIO COMMITTEE ON DEFENCE AND MILITARY VETERANS PARLIAMENT
- RESEARCHING DIVERSITY IN THE SCIENTIFIC WORKFORCE CAN BE CHALLENGING
- PARTAI POLITIK ALTERNATIF DAN PEMILU HINGGA SAAT INI KEKUATAN
- CONTESTACIÓN DE DEMANDA DE NULIDAD DE BOLETA DE INFRACCIÓN
- THE MARYLAND STATE PARENTAL INFORMATION RESOURCE CENTER (PIRC) IS
- CSO1058AS (817) ARIZONA DEPARTMENT OF CHILD SAFETY CHILD SAFETY
- PŘÍLOHA ÚPLNÉ ZNĚNÍ INTEGROVANÉHO POVOLENÍ (ČJ 24537ZP06MTP ZE DNE
- (R 404) TIP SHEET ON FAMILY ENGAGEMENT THE GOAL
- WTMIN(01)ST150 PÁGINA 3 ORGANIZACIÓN MUNDIAL DEL COMERCIO WTMIN(01)ST150 12
- THIS VERSION OF THE PLANNING PRACTICE NOTE 57 THE
- THE 2014 DBA UK FAMILY WEEKEND FRIDAY
- INCREASED ADAPTABILITY OF YOUNG JUDO SPORTSMEN AFTER PROTEIN SUPPLEMENTATION
- GUILLERMO ORTEGA ECONOMISTA UCV 1984 BECARIO FULLBRIGHT 19891993 ESTUDIOS
- 15A NCAC 02B 0604 SITE SPECIFIC WATER QUALITY MANAGEMENT
- R COMUNICADO DE PRENSA URAL DEVELOPMENT 654 PLAZA SUITE
- 4 DUNAJSKÁ STREDA DŇA 05022016 1SPRO192016 OZNÁMENIE O VYHLÁSENÍ
- İSTANBUL KÜLTÜR VE SANAT ÜRÜNLERİ TİC AŞ’DEN YENİ
- INCIDENT DETAILS NAME OF PERSON INVOLVED IN THE INCIDENT
- WORCESTER CHILD DEVELOPMENT HEAD START PROGRAMWPS 770
- BIDATZA 503 ZK 2021EKO URRIA SIERRA DE ENTZIA
- PROJECTE GLOBAL D’EMPRENEDORIA DE LA UNIVERSITAT DE GIRONA 1
- WNIOSEK O PRZEKAZYWANIE WYPŁATY ŚWIADCZEŃ NA RACHUNEK BANKOWY DANE
- MARYLAND TELECOMMUNICATIONS STAFF CHECKLIST FOR REVIEWING TARIFFS COMPANY NAME
- RISTOURNE À IMPÔT DIFFÉRÉ ATTESTATION DES ADMINISTRATEURS OU DIRIGEANTS
 DIABETIC RETINOPATHY PATHWAYS THESE ARE NOT YET IMPLEMENTED
DIABETIC RETINOPATHY PATHWAYS THESE ARE NOT YET IMPLEMENTED RESOLUÇÃO Nº 215 DE 14 DE DEZEMBRO DE 2006
RESOLUÇÃO Nº 215 DE 14 DE DEZEMBRO DE 2006AS 20192020 PROGETTO “ORIENTARE AL SUCCESSO FORMATIVO”
ZÁPIS Z E ZASEDÁNÍ ZASTUPITELSTVA MĚSTSKÉ ČÁSTI BRNO –
 OCTOBER 2017 ADVANCE PITSTOP CORPORATE CLUB – FREQUENTLY ASKED
OCTOBER 2017 ADVANCE PITSTOP CORPORATE CLUB – FREQUENTLY ASKED ÁREA DE ORIENTACIÓN Y COORDINACIÓN DE PRÁCTICAS EN EMPRESA
ÁREA DE ORIENTACIÓN Y COORDINACIÓN DE PRÁCTICAS EN EMPRESAGINÉS CIUDADREAL NÚÑEZ TÉCNICAS DE COOPERATIVO TALLER DE ESCRITOR
TERMINY POSTĘPOWANIA REKRUTACYJNEGO ORAZ POSTĘPOWANIA UZUPEŁNIAJĄCEGO A TAKŻE TERMINY
 PROCEDURA DOKONYWANIA OCENY RODZINY ZASTĘPCZEJ ORAZ PROWADZĄCEGO RODZINNY DOM
PROCEDURA DOKONYWANIA OCENY RODZINY ZASTĘPCZEJ ORAZ PROWADZĄCEGO RODZINNY DOM1 MEDFAKULTETNI DOKTORSKI ŠTUDIJSKI PROGRAM EPISTEMOLOGIJA HUMANISTIKE IN DRUŽBOSLOVJA
HAND SPECIMEN MINERALOGY LAB OXIDES FOR MINERALS IN
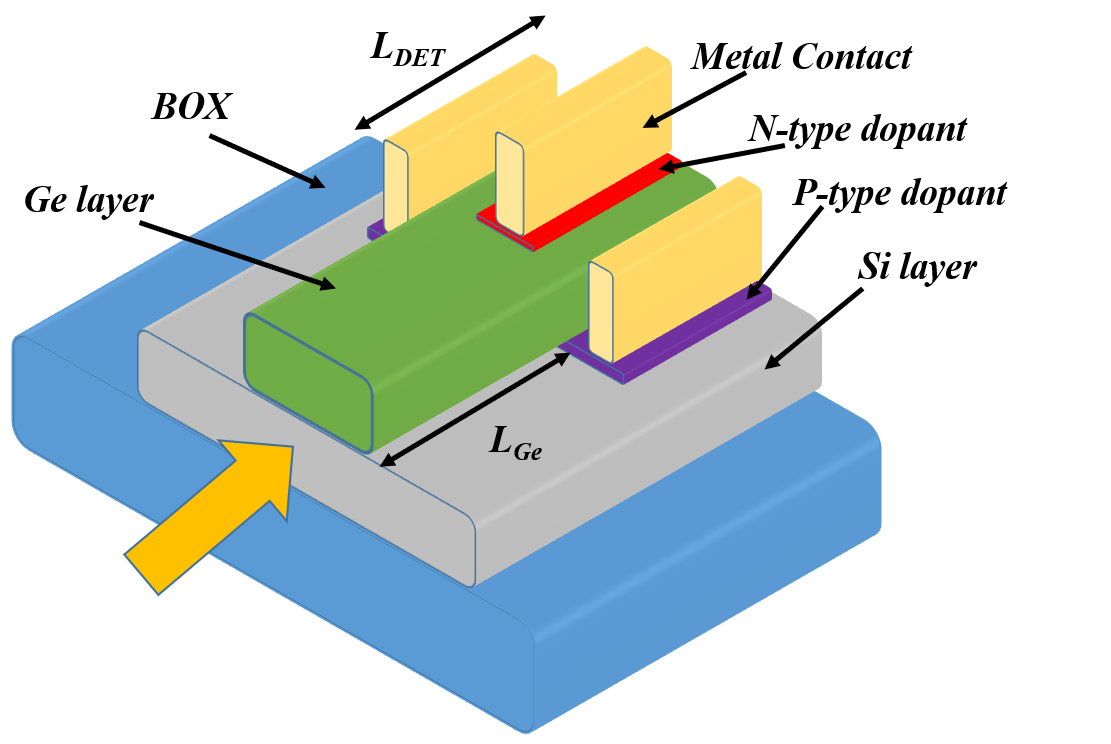 7 REPLACE THIS LINE WITH YOUR PAPER IDENTIFICATION
7 REPLACE THIS LINE WITH YOUR PAPER IDENTIFICATION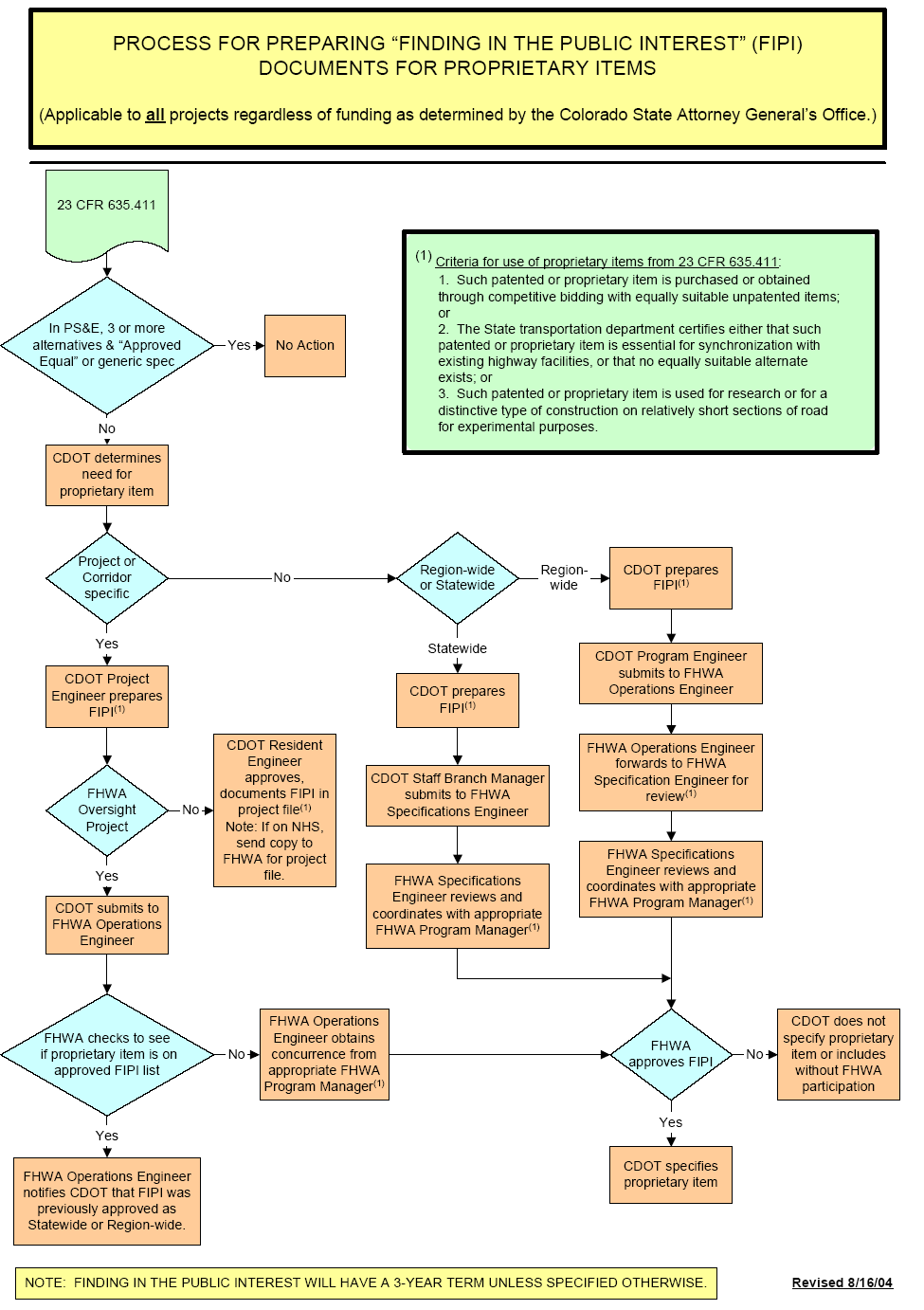 GENERAL JULY 2001 [REVISED AUGUST 2004] 816 PROPRIETARY ITEMS
GENERAL JULY 2001 [REVISED AUGUST 2004] 816 PROPRIETARY ITEMSText Version of Gunnar Hellstrom’s Power Point Presentation Most
BARNE UNGDOMS OG FAMILIEETATEN 6 (BESVARES OG TAS MED
 PODER JUDICIAL DE TUCUMÁN PLIEGO UNICO DE BASES Y
PODER JUDICIAL DE TUCUMÁN PLIEGO UNICO DE BASES Y APPENDIX 5 QUOTA MONITORING PERMITS REPORTING FORM (LOG)
APPENDIX 5 QUOTA MONITORING PERMITS REPORTING FORM (LOG) V ICERRECTORADO DE INVESTIGACIÓN CONSEJERÍA DE INNOVACIÓN CIENCIA Y
V ICERRECTORADO DE INVESTIGACIÓN CONSEJERÍA DE INNOVACIÓN CIENCIA Y COMUNICACIÓ DE CREMA A ZONA URBANA PERÍODE COMPRÈS ENTRE
COMUNICACIÓ DE CREMA A ZONA URBANA PERÍODE COMPRÈS ENTRE CONVOCATORIA DEL CONCURSO DE BECAS DE EDUCACIÓN ESPECIAL CURSO
CONVOCATORIA DEL CONCURSO DE BECAS DE EDUCACIÓN ESPECIAL CURSO