DOMESTICATION OF ALLANBLACKIA FLORIBUNDA VEGETATIVE PROPAGATION BY LEAFY STEM
13 SPERM COMPETITION AS AN UNDERAPPRECIATED FACTOR IN DOMESTICATION34 A REVIEW OF DOMESTICATION EFFECTS ON STOCKED FISHES
DOMESTICATION OF ALLANBLACKIA FLORIBUNDA VEGETATIVE PROPAGATION BY LEAFY STEM
INSTRUCTIONSDOMESTICATION OF A FOREIGN CORPORATION TO THE MARSHALL ISLANDS
Domestication of Allanblackia floribunda: vegetative propagation by leafy stem cuttings in the Niger Delta Region of Nigeria
DOMESTICATION OF ALLANBLACKIA FLORIBUNDA: VEGETATIVE PROPAGATION BY LEAFY STEM CUTTINGS IN THE NIGER DELTA REGION OF NIGERIA
1*Anegbeh, P. O., 2Tchoundjeu, Z., 3Simons, A. J. and 4Roy-Macauley, H.
1 World Agroforestry Centre (ICRAF), West and Central Africa, ICRAF Office, c/o International Institute of Tropical Agriculture (IITA), IITA Station, Onne, P.M.B. 008, Nchia-Eleme, Rivers State, Nigeria. Tel: +234 2 241 2626 Ext 2399. Fax: +234 2 241 2221. Email: [email protected] 2World Agroforestry Centre (ICRAF), West and Central Africa, Yaounde, Cameroon. 3World Agroforestry Centre (ICRAF), East and Central Africa, P. O. Box 30677, Nairobi, Kenya, 4World Agroforestry Centre (ICRAF), West and Central Africa, BP 320, Bamako, Mali.
* Corresponding Author
Running Title: Domestication of Allanblackia in Nigeria Anegbeh et al.
ABSTRACT
Allanblackia floribunda, one of the indigenous fruit tree species of West, Central, and East Africa, is in danger of extinction. In spite of its uses as livestock feed, bait to trap animals, firewood etc, the species had in the past received little attention. Information on the vegetative propagation of A. floribunda is currently unavailable in published forms. Studies were carried out at ICRAF nursery located at Onne, Rivers State, Nigeria to determine the rooting success of single node leafy stem cuttings of A. floribunda. In 2005, four fruiting female trees were selected from farmers’ fields located at Onne. The treatments consisted of softwood cuttings (re-growth) collected from three felled female trees marked as T1, T2, and T3, and hardwood cuttings collected from canopy of mature tree (control), marked as T4. The cuttings were trimmed and placed on white river sand in non-mist propagators for 11 months. Rooted cuttings were potted, gradually hardened off, allowed to initiate new growth and planted out in a gene bank. Results of the experiment demonstrated that rooting ability of A. floribunda basically depended on genotypes. Cuttings set on river sand substrate took 3 months to root. The best cultivar with the most rooting ability had over 50% rooting in 8 months. This is encouraging since seeds of A. floribunda require 8 to 12 months to achieve 0.01% germination. There was significant linear relationship between rooting percent and time in all four trees. This information is important as it helps to encourage farmers to domesticate A. floribunda. Trees 2 and 3 (T2 and T3) are recommended to farmers. Participatory tree domestication is being used in trees and market theme of ICRAF to develop A. floribunda and other fruit and medicinal trees for rural farmers in an effort to contribute to the overall nutritional well being and reduce poverty of rural communities in Africa.
Keywords: Allanblackia floribunda, cuttings, Niger Delta, rooting, softwood
INTRODUCTION
Domesticating agroforestry trees involves accelerated and human-induced evolution to bring species into wider cultivation through farmer-driven and market-led process. Its use and adoption enable rural farmers to commercially produce improved propagules of trees, especially fruit trees.
Allanblackia, named after Allan Black, 19th century key botanist is a small genus. Allanblackia floribunda Oliv is one of the nine species of the genus Allanblackia. The tree, which grows up to 30 m, is evergreen, and has a straight bole (occasionally fluted). Branches of the tree are slender and dropping and often conspicuously whorled (Keay, 1989). It belongs to the family Clusiacea.
The fruit of A. floribunda, which is known as izeni and uzoka (Edo), orogbo-erin (Yoruba), egba (Ibo), ediang (Efik), obobio-obo (Ijaw), contains about 50 brittle-shelled seeds. The fruit is brown, roughly fleshy and slightly grooved longitudinally. It is often confused with Pentadesma butyracea and Garcinia kola.
Ecologically, Allanblackia floribunda is found in wide range of habitats: it is distributed in wet evergreen rainforests. The tree thrives well in the Niger Delta Region of Nigeria, especially in abandoned forests (acidic soils) with rainfall as high as 2400 mm. It is also found in forest reserves, fallow lands, surrounding farmlands, etc.
Continentally, Allanblackia floribunda is found in many African countries (Angola, Cameroon, Democratic Republic of Congo, Ghana, Kenya, Nigeria, Sierra Leone, and Tanzania).
It is a seasonal fruit and mostly found during the rainy season. It is an indigenous fruit tree that has been recognized recently as containing valuable industrial edible oil, which is used for the manufacturing of margarine, soap, chocolate, ointment and food products (cashew and pea nuts).
Ethnobotanic studies on the traditional uses of Allanblackia floribunda carried out in Nigeria in 2005 clearly indicated that farmers valued Allanblackia floribunda for firewood and for making huts, doors, windows, poles, bridge-piles, yam stakes, candlesticks, chewing sticks. Local hunters use the fruits as animal feeds/bait in trapping animals such as rats and porcupines (Anegbeh et al 2005a). Cooked seeds are edible in Gabon. Kernels have 25.5% stearic acid and 46.9% oleic acid. Medicinally, the bark relieves body pain, cough, asthma, bronchitis, dysentery and tooth ache.
The germination of A. floribunda seeds is problematic. Untreated seeds take 8 to 12 months to reach 0.01% germination. Seeds treated with gibberellic acid did not germinate after 7 months of testing. It appears, therefore, that alternative approaches need to be developed urgently for propagating A. floribunda. One such approach is vegetative propagation by leafy stem cuttings. But no published data are available on the vegetative propagation of A. floribunda in Nigeria. This study was conducted to determine the rooting ability of different genotypes of A. floribunda propagated by leafy stem cuttings in the Niger Delta Region of Nigeria.
MATERIALS AND METHODS
The study was conducted from 2005 to 2006 at ICRAF tree nursery and demonstration fields located at the International Institute of Tropical Agriculture (IITA), Onne, (040 51' N Latitude 070 03' E Longitude), Rivers State. The station has annual rainfall of 2400 mm (Anegbeh et al., 2006).
In 2005, superior trees with desirable traits were selected from fruit bearing trees in fallow and farmers’ fields at Onne village and marked. They were given identification numbers as T1, T2, T3, and T4. Three of the selected trees (T1, T2, and T3) were felled to obtain re-growth (coppicing). Non-mist propagators were constructed in the nursery and filled with different substrates.
Cuttings of A. floribunda were collected from the selected female trees during the last quarter of the year 2005 when most of the fruits have been collected. The selected cuttings were free-from diseases and insect pests. A minimum of four hundred cuttings of A. floribunda of known identity were selected from each of the mature female parent trees and dipped in a bucket of water prior to moving into the nursery.
The cuttings were prepared using standard procedure developed by ICRAF. Pair of scissors and surgical blade were used to trim the leaves of the cuttings and inserted inside the substrate, containing white river sand, in the non-mist propagators. A knapsack sprayer was used to water the cuttings. Rooting of cutting was monitored at one month interval. The treatments consisted of softwood cuttings (re-growth) collected from three genotypes of felled female trees marked as T1, T2, and T3, and hardwood cuttings collected from canopy of mature tree (control), marked as T4.
The rooted cuttings were removed from the non-mist propagators when well-developed adventitious roots were observed. Harvested cuttings were potted directly inside black polyethylene bags measuring 25 x 35 x 45 cm which were filled with potting mixture (top soil, sawdust and sand) in the ratio of 3: 2: 1. They were arranged inside giant humidity chambers. Watering of the cutting was done to maintain high humidity environment, between 75 and 100%, within the chambers, by applying about 10 cl of water to each plant twice daily for one week. Thereafter, watering was done once a day.
The rooted cuttings were hardened for three months in a partially shaded condition (acclimatization). The rooted and weaned plants of A. floribunda were planted in field gene bank in September 2006 at 10 m x 8 m spacing (125 plants per hectare). The cuttings were replicated three times in a completely randomized design Analysis of variance (ANOVA) and regression analysis were conducted using statistical analysis system (SAS) program package (SAS 1996).
RESULTS
No detectable rooting was observed during the first 2 months after setting (2 MAS) the cuttings in all of the cultivars evaluated. At 3 months of setting, small percentages of rooting were obtained for T1, T2, and T3 (Fig 1).
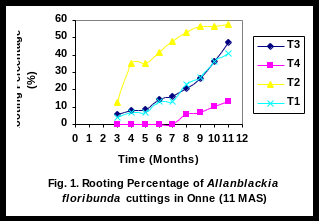
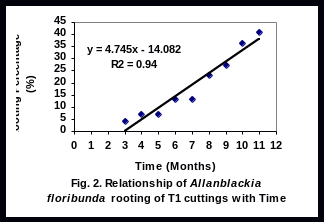
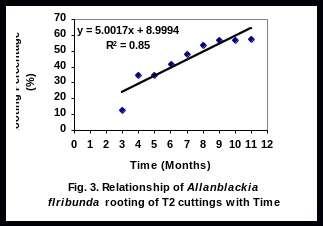
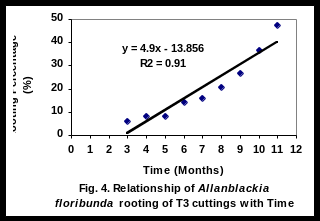
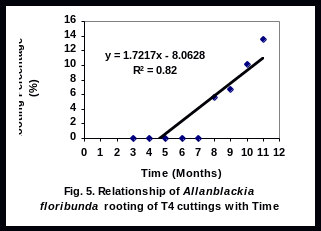
There was a significant linear relationship (R2 = 0.94), (R2 = 0.85), (R2 = 0.91), and (R2 = 0.81), between rooting percent of leafy stem cuttings and time for T1, T2, T3 and T4 respectively (Figs 2-5). Rooting percentage of plants of T2 increased linearly with increasing period of time. All rooted cuttings survived transplanting to polyethylene bags.
DISCUSSION
Rooting of T4 (control plants) commenced at eight months after setting and the percentage rooting remained low throughout the experimental period (Fig 1). The reason for this low performance is unknown, but might have been caused by specific characters of the cultivar (Tchoundjeu and Leakey 2001). Nevertheless, rooted cuttings maintained their healthy appearance at the end of the experiment. Also, they are valuable as superior materials in tree domestication as they possess desirable traits.
Cuttings of T2 displayed an increase in percent rooting, possibly indicating that young tree responds better than old tree. The continuing increase in percent rooting for T2 plants indicates that the cultivar is promising for integrating into farming systems in the Niger Delta Region. The percentage rooting of T1 and T3 remained low until 6 month, when a sharp increased was obtained.
The rate of rooting in T2 dropped rapidly from 6 to 7 months after setting. Since a high percent rooting was obtained 6 months after setting for T2, a comparable percent rooting was also expected 6 months after setting the other trees. However the other trees rooted mostly at 8 months after setting (8 MAS). Forty percent of leafy stem cuttings of T2 rooted within 4 months without auxin application. These results are similar to those of other tropical trees (Tchoundjeu and Leakey, 2001). Except for T4, cuttings of the trees commenced rooting at three months after setting (3 MAS).
The ability of Allanblackia floribunda to respond positively to vegetative propagation is comparable to that of Dacryodes edulis, an edible fruit tree (Anegbeh et al, 2005b). The ability of T2 to outperform T1, T3 and T4 apparently may be due to more rapid developmental changes and elongation of cells in the softwood of the youngest tree (T2), following the felling and re-sprouting of the shoot, than the other trees (T1, T3, and T4).
Thus the rapid rooting of T2 may be an indication of the quality of softwood cuttings of the young tree. This is also the view of Pijut and Moore (2002). Since early rooting is desirable for tree domestication, T2 and T3 cultivars are appropriate materials for agroforestry farmers.
The significant linear relationship between rooting percent and time of T2 cultivar is important as it helps to calculate expected plant production at any given time. It is doubtful whether farmers will wait for over one year for Allanblackia seeds to germinate. This discouragingly long period required for seeds of A. floribunda to germinate together with low rate of germination is one of the constraints to Allanblackia domestication. In light of this experiment, rooting ability tested for 11 months, results have helped to select some cultivars (T2 and T3) promising for domesticating Allanblackia floribunda.
CONCLUSIONS
Participatory tree domestication has been used by ICRAF Researchers to identify, collect germplasm, propagate and integrate high-value indigenous fruit and medicinal trees into farming systems (Simons, 1996; Anegbeh, et al., 2006). Propagation by vegetative means is often the best way to preserve selected traits in trees. When seeds are in short supply, unavailable, difficult to germinate, or sterile (Shi and Brewbaker, 2006), vegetative propagation may be the only means available. This study enabled an accurate understanding of vegetative propagation of A. floribunda, which could benefit resources-poor farmers and ensure judicious domestication of the species.
The vegetative propagation by leafy stem cuttings used in this study showed that the most rooting ability of the trees was T2. This was followed by T3. The germplasm of T2 and T3 will be useful for the development of cultivars of the most important fruit traits.
The experiment clearly shows that vegetative propagation by leafy stem cuttings can increase the production of Allanblackia floribunda planting materials. The use of root promoting hormones (such as indole-3- butyric acid) and low-technology approach may further help increase the possibility of producing planting materials as observed for Inga feuillei cuttings (Brennan and Mudge, 1998).
Recognizing the enormous potential of Allanblackia floribunda in poverty reduction and food security, the World Agroforestry Centre (ICRAF) together with national agricultural research and extension systems (NARES) is domesticating Allanblackia in Nigeria, Kenya, Cameroon, Ghana and Tanzania.
ACKNOWLEDGEMENT
The Authors thank all farmers working with ICRAF in Nigeria and beyond and Unilever (Netherlands) who guaranteed international market for Allanblackia. This effort has attracted the attention of diverse groups to the species.
REFERENCES
Anegbeh, P. O., Tchoundjeu, Z. and Simons, A. J. 2005a. Update on domestication of Allanblackia in Nigeria. Paper Presented at Allanblackia Planning Meeting. Seminar Room, ICRAF Headquarters, Nairobi, Kenya. 27 Nov – 4 Dec 2005. 12p.
Anegbeh, P. O., Ladipo, D. O. and Tchoundjeu, Z. 2005b. Using marcotting technique for fruit development in the African pear Dacryodes edulis. Scientia Africana 4 (1 & 2): 102-108.
Anegbeh, P. O., Iruka, C. and Nkirika, C. 2006. Enhancing germination of bitter cola (Garcinia kola), heckel: prospects for agroforestry farmers in the Niger Delta. Scientia Africana 5 (1): 38-44.
Brennan, E. B. and Mudge, K. W. 1998. Vegetative propagation of Inga feuillei from shoot cuttings and air layering. New Forests. 15(1): 37-51.
Keay, R. W. J. 1989. Trees of Nigerian. A Reverse Version of Nigeria Trees. Clarendo Press Oxford.
Pijut, P. M. and Moore, M. 2002. Early Season Softwood Cuttings Effctive for Vegetative Propagation of Juglans cinerea. HortScience 37(4): 697-700.
SAS, 1996. Statistical Analysis Systems. SAS procedures guide for personal computer, Release 6.12 edition. SAS Incorporation, Cary NC. USA. 112 p.
Shi, X. and Brewbaker, J. L. 2006. Vegetative propagation of Leucaena Hybrids by cuttings. Agroforestry Systems. 66(1): 77-83.
Simons A.J. 1996 ICRAF’s strategy for domestication of indigenous tree species. In: Domestication and Commercialization of Non-timber Forest Products in Agroforestry Systems. FAO Special Publication, Forest Division, FAO, Rome. P8-22.
Tchoundjeu, Z and Leakey R. R. B. 2001. Vegetative propagation of Lovoa trichilinoides: effect of provenance, substrate, auxins and leaf area. Journal of Tropical Forest Science 13 (1): 116-129.
Tags: allanblackia floribunda:, at allanblackia, leafy, floribunda, allanblackia, propagation, vegetative, domestication
- MANUAL GENERAL DE DEBERES POLICIALES DE SEGURIDAD PÚBLICA DEL
- WARWICK DISTRICT COUNCIL LOCAL PLAN MAIN MODIFICATIONS SUSTAINABILITY APPRAISAL
- UNITED NATIONS ECONOMIC COMMISSION FOR EUROPE COMMITTEE ON TRADE
- STUDENTS WITH VISUAL IMPAIRMENTS IN HIGHER EDUCATION INSTITUTES KOUTSOKLENIS
- EL ADVERBIO DI EN QUÉ ORACIONES LAS PALABRAS SUBRAYADAS
- AREMA COMMITTEE 10 MEETING MINUTES JUNE 17 & 18
- 8 PL170627 LOCAL PLANNING APPEAL TRIBUNAL TRIBUNAL D’APPEL DE
- 8 HACIA UNA PREVIA AUNQUE FUTURA CONFIGURACIÓN DEL CONVENIO
- FORMA PATVIRTINTA AKMENĖS RAJONO SAVIVALDYBĖS MERO 2019 M VASARIO
- ACUERDO 432021 DE 26 DE ABRIL DE LA JUNTA
- 2014 ANGIE’S LIST SUPER SERVICE AWARD® TRADEMARK USAGE STANDARDS
- URZĘDÓW 12062018R INFORMACJA Z OTWARCIA OFERT DOT POSTĘPOWANIA O
- ZAŁĄCZNIK DO UCHWAŁY SENATU NR792018 Z DNIA 28062018R KARTA
- APPLICATION FOR FUNDING FORM YOU MUST COMPLETE ALL SECTIONS
- WALSALL TOWN OF A HUNDRED TRADES WALSALL HAS BEEN
- LAB 2 PROBABILITY DISTRIBUTIONS LMFE 2006 GOALS THE GOALS
- TERMO DE COMPROMISSO DE ESTÁGIO EXTRACURRICULAR (NÃOOBRIGATÓRIO) A
- 30 PEDOMAN PENULISAN SKRIPSI FAKULTAS TEKNIK DAN ILMU KELAUTAN
- I FRIQIYYA ELECTRIQUE RIDERS TECHNICAL RIDER STAGE
- WWWRECURSOSDIDACTICOSORG APARATO DIGESTIVO TUBO DIGESTIVO BOCA
- PLAZA DE LA CONSTITUCIÓN 1 30850 TOTANA (MURCIA) TELF
- PRESUPUESTO POR ACTIVIDADES NÚMERO DE EXPEDIENTE ACTIVIDADES PORCENTAJE DESTINADO
- 275 VYHLÁŠKA ZE DNE 12 ŘÍJNA 2015 KTEROU SE
- LANGVERSION (4241 ZEICHEN) AUF SCHNELLEN ROLLEN DURCH DEN FLÄMING
- HOUSE COMMITTEE ON VETERANS’ AFFAIRS SUBCOMMITTEE ON DISABILITY
- 27 LOGO EMPRESA PLAN DE NEGOCIOS (NOMBRE EMPRESA)
- IA ÉN ANNÓ EZT HASZNÁLTAM PL (MŰEGYETEMI EVEZŐS KLUB
- APPLYING FOR VETERANS’ EDUCATIONAL BENEFITS MOST FULL TIME CAREER
- GP PATIENT SURVEY PUBLISHED BY NHS ENGLAND USER FEEDBACK
- TECNOLOGIA IES “GONZALO ANAYA” XIRIVELLA NOMBRE GRUPO ACTIVIDAD
 ALAMEDA COUNTY BEHAVIORAL HEALTH CARE SERVICES SERVICE DATE MENTAL
ALAMEDA COUNTY BEHAVIORAL HEALTH CARE SERVICES SERVICE DATE MENTALTALLER DE ESCRITURA CREATIVA ANIMADO POR SYLVIE NYS
ORDEN DE XX DE JULIO DE 2012 DE LA
 RECOMMENDATION (REVISED) TO NAESB EXECUTIVE COMMITTEE FOR QUADRANT WEQ
RECOMMENDATION (REVISED) TO NAESB EXECUTIVE COMMITTEE FOR QUADRANT WEQDANE IDENTYFIKACYJNE DOKTORANTA NAZWISKO IMIĘ DRUGIE IMIĘ NAZWISKO RODOWE
EVEN VOORSTELLEN RUIM 15 JAAR GELEDEN NAMEN WIJ HET
 CONTRAT DE TRAVAIL A DUREE INDETERMINEE A TEMPS PLEIN
CONTRAT DE TRAVAIL A DUREE INDETERMINEE A TEMPS PLEIN RESOLUCIÓN DE 15 DE DICIEMBRE DE 2009 DEL RECTOR
RESOLUCIÓN DE 15 DE DICIEMBRE DE 2009 DEL RECTORSLOW FOOD POST EVENT REPORT TO BOARD REPORT DATE
 SALAZAR CONFIGURACIÓN DEL NOSOTROS Y DEL OTRO APROXIMACIÓN A
SALAZAR CONFIGURACIÓN DEL NOSOTROS Y DEL OTRO APROXIMACIÓN ALEI MUNICIPAL Nº 628092012 DE 15 DE MAIO DE
 VILNIAUS UNIVERSITETAS LIETUVOS PSICHOLOGŲ SĄJUNGA IV PASAULIO LIETUVIŲ PSICHOLOGŲ
VILNIAUS UNIVERSITETAS LIETUVOS PSICHOLOGŲ SĄJUNGA IV PASAULIO LIETUVIŲ PSICHOLOGŲ24 CINCO MEGATENDENCIAS DEL PROCESO DE GLOBALIZACIÓN SENTIDO BIOÉTICO
PRESSEINVITASJON STATOIL OG NTNU INNGÅR AVTALE OM FORSKNINGSPROFESSORATER OG
STUDY ON THE ACTION PLAN FOR LIVABLE BAY AREA
CURRICULUM VITAE ALBERTO MUÑOZ TEROL LUGAR Y FECHA
 VICERRECTORADO DE RELACIONES INTERNACIONALES CERTIFICADO DE LLEGADA ARRIVAL CERTIFICATE
VICERRECTORADO DE RELACIONES INTERNACIONALES CERTIFICADO DE LLEGADA ARRIVAL CERTIFICATEPRECONQUEST ALPHABETICAL LIST FOR SOURCES ENTER NAME IN DATABASE
INDEPENDENT READING ACTIVITIES PICK A DESCRIPTIVE WORD FROM THE
 DRUŠTVO MEPI MEDNARODNO PRIZNANJE T +386 (0)41 926
DRUŠTVO MEPI MEDNARODNO PRIZNANJE T +386 (0)41 926