DOMINANCE OF LONGCHAIN NODIACYLATED ETHANOLAMINE IN MIXED AMPHIPHILIC ACID
6 DOMINANCE A DIVERZIFIKACE OBCHODNÍCH FIREM V RÁMCISOCIAL REVERSAL OF SEXBIASED AGGRESSION AND DOMINANCE IN A
CHOOSING AMONG RISKY ALTERNATIVES USING STOCHASTIC DOMINANCE WHEN IS
DOMINANCE OF LONGCHAIN NODIACYLATED ETHANOLAMINE IN MIXED AMPHIPHILIC ACID
DOMINANCE THEORY – CURRENT THINKING DOGS TRUST IS THE
LIST OF PARTICIPANTS TO THE TRAINING WORKSHOP “ABUSING DOMINANCE
Amphiphilic derivatives of ethanolamine attracted attention not only because of their occurrence in a wide variety of animals, plants, and microbes []- but also their interesting biological, pharmaceutical and medicinal properties []
Dominance of Long-chain N,O-Diacylated Ethanolamine in Mixed Amphiphilic Acid Amide Monolayers
G. Brezesinski1, I. Berndt1, B. Dobner2, D. Vollhardt1*
1Max Planck Institute of Colloids and Interfaces, D-14424 Potsdam/Golm, Germany
2University Halle-Wittenberg, Institute of Pharmacy, D-06120 Halle, Saale Germany
Keywords: monolayers; binary and ternary mixtures; acid amide amphiphiles, grazing X-ray diffraction, Brewster angle microscopy
Abstract
The main characteristics of Langmuir monolayers of acid amide amphiphiles with one and two alkyl chains are fundamentally different. This is demonstrated by comparison of surface pressure – area (p-A) isotherms, the mesoscopic morphology, and the two-dimensional lattice structure of the condensed phases in the case of the single-chain acid amides with N-myristoyl ethanolamine (TDAHA) and 3-hydroxy-N-tridecyl propanoic acid amide (HTPA) as well as in the case of the double-chain acid amide with N,O-dimyristoyl ethanolamine (TAOAE). The studies of the binary mixtures TDAHA/TAOAE, HTPA/TDAHA, HTPA/TAOAE (1:1) and ternary mixtures TAOAE/TDAHA/HTPA (1:1:1) performed at 5 °C and 25 °C allow the comparison with the single components. The results indicate convincingly that all binary 1:1 monolayers and the ternary 1:1:1 monolayer exist as homogeneously mixed systems. The dominance of the double-chain TAOAE in the binary and ternary monolayers mixed with single-chain acid amide amphiphiles TDAHA and HTPA in mesoscopic and molecular scale can be unequivocally concluded from the GIXD and BAM measurements. The probability for the presence of N,O-diacylated ethanolamine, such as TAOAE, as impurity in N-acylated ethanolamine, e.g. TDAHA, is high so that in such systems the most important interfacial characteristics are dominated by the impurity.
Introduction
A variety of papers has demonstrated that the chemical structure of the amphiphiles determines largely the main characteristics of their Langmuir monolayers [1,2]. Even small alterations in the molecular structure of the amphiphile can change the phase behaviour and packing properties of the condensed-phase monolayers and can substantially affect the main characteristics of the monolayer, in particular the surface pressure-area per molecule (π-A) isotherms, the mesoscopic morphology, and the two-dimensional lattice structure of the condensed phase [3-8].
In biological systems, the role of the amide group as integral part of sphingolipid is of special interest [9,10]. Therefore biomimetic monolayer studies have focused onto adequate model amphiphiles containing amide and amine [9-11]. Particularly, amphiphilic derivatives of ethanolamine attracted attention not only because of their occurrence in a wide variety of animals, plants, and microbes [12,13], but also because of their interesting biological, pharmaceutical and medicinal properties [14-17].
According to the significance of N-acylethanolamines, the effect of the position of the substituents at the amide group on the major monolayer characteristics was studied in a previous work. For this purpose, the monolayer characteristics of two similar amphiphiles N-myristoyl-ethanolamine (C13H27-CO-NH-C2H4OH; HETA) and 3-hydroxy-N-tridecyl propanoic acid amide (C13H27-NH-CO-C2H4OH; HTPA) [18], different in the chemical structure only in the position of the two substituents at the amide group, were compared [19].
Renewed synthesis and careful analysis of the two amphiphiles have shown that the N-myristoylethanolamine (HETA) sample used in ref. [19] contained a second amphiphilic component as impurity. As ethanolamine having an amino group and a hydroxy group offers the possibility for N- and O-acylation [20,21]. The corresponding N,O-diacyl derivative of ethanolamine tetradecanoic acid-2-[(1-oxotetradecyl) amino]ethyl ester (C13H27-CO-NH-(CH2)2-O-CO-C13H27, TAOAE) can be expected to be the amphiphilic impurity of the HETA sample used in Ref. [19]. To clarify the situation and the role of N,O-diacyl derivatives of ethanolamine as impurity the main characteristics, namely surface pressure – area (p-A) isotherms, morphology of the condensed phase domains, lattice structure of the condensed phase and the existence of hydrogen bonds (-NH· · ·O=C-) of the monolayers of a highly purified N-myristoyl ethanolamine (C13H27-CO-NH-C2H4OH, TDAHA) and the corresponding N,O-diacyl derivative of ethanolamine tetradecanoic acid-2-[(1-oxotetradecyl) amino]ethyl ester (TAOAE) have been determined [22,23]. The comparison of the p-A isotherms of TDAHA and TAOAE suggests the larger surface activity of TAOAE indicating a dominant effect if present as impurity in TDAHA monolayers (e.g. the HETA component in ref. [19].
The possible effect of surface-active impurities on the characteristics of the main component is a known phenomenon. The most representative example of such an effect is the dominance of dodecanol traces in the characteristics of the main component sodium dodecylsulfate [24,25]. The objective of the present work is to compare the monolayer characteristics of all three pure amphiphilic amides TAOAE, TDAHA and HTPA (Figure 1) and their mixtures TDAHA/TAOAE, HTPA/TDAHA, HTPA/TAOAE (1:1) and TAOAE/TDAHA/HTPA (1:1:1) to obtain first information about the resulting characteristics in these mixtures of the components. For this reason, characteristic feature of these mixtures are studied at temperatures at which interesting information can be expected.
Experimental
Materials
The amphiphilic amides TDAHA [22], TAOAE [21] and HTPA [19] were synthesized as previously described in detail. The chemical purity of all amphiphiles was checked by elemental analysis, 1HNMR, column chromatography (TDAHA; TAOAE) and HPLC (HTPA).
The used spreading solvent was chloroform (p.a. grade, Baker, Holland). Ultrapure water produced by “Purelab Plus” was used as subphase.
Surface pressure measurements and Brewster angle microscopy
An experimental setup consisting of a self-made computer interfaced film balance coupled with a Brewster angle microscope (BAM1+, NFT, Göttingen, Germany) was used to measure the equilibrium surface pressure (π-A) isotherms at a compression rate of 10 Å2/(molecule·min) [26]. Using the Wilhelmy method, the surface pressure was measured with a roughened glass plate with an accuracy of 0.1 mN·m-1, and the area per molecule with 0.5 Å2. The lateral resolution of the BAM1+ was approximately 4 m. Simple imaging processing software was used to improve the contrast. Detailed information about the BAM method is given elsewhere (see refs. 27-29).
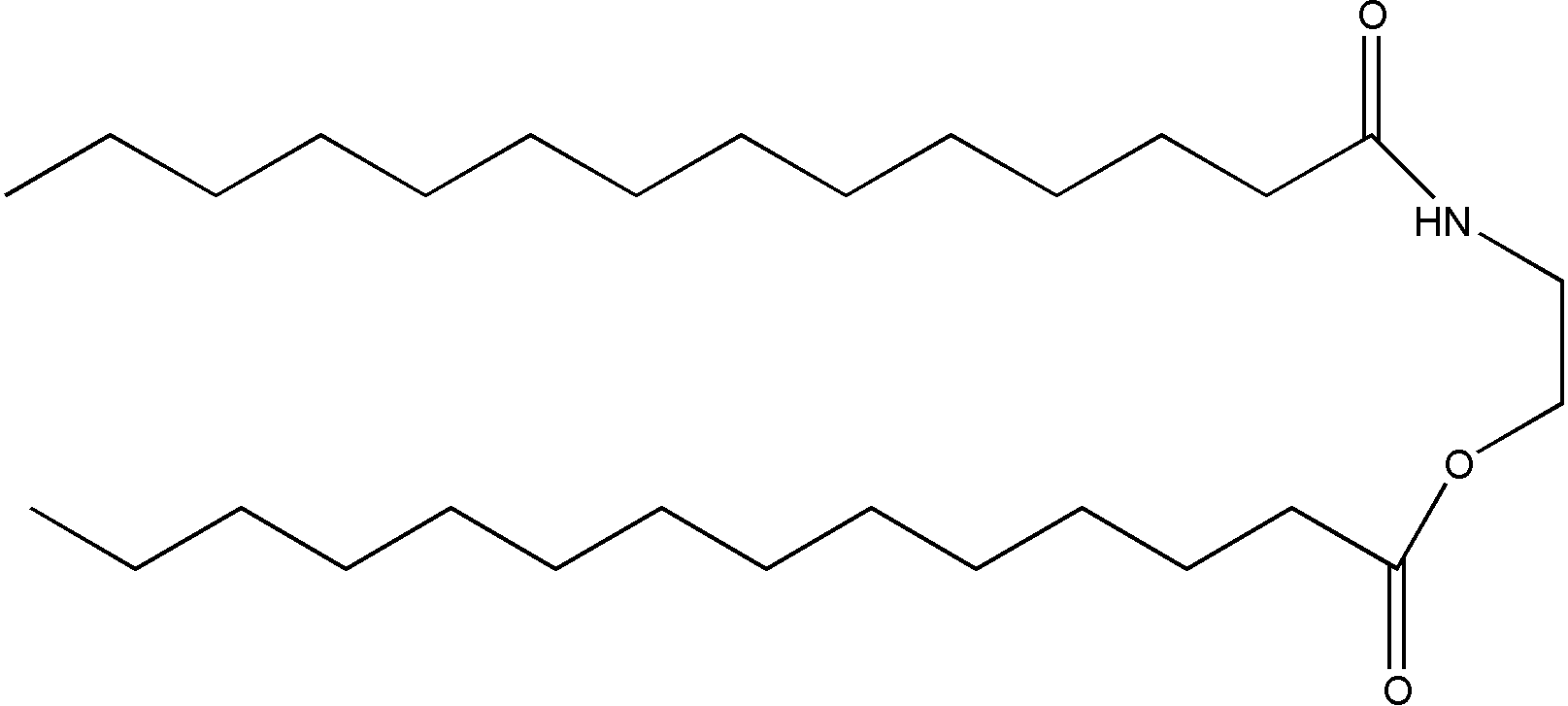
Tetradecanoic acid-2-[(1-oxotetradecyl) amino]ethyl ester (TAOAE)
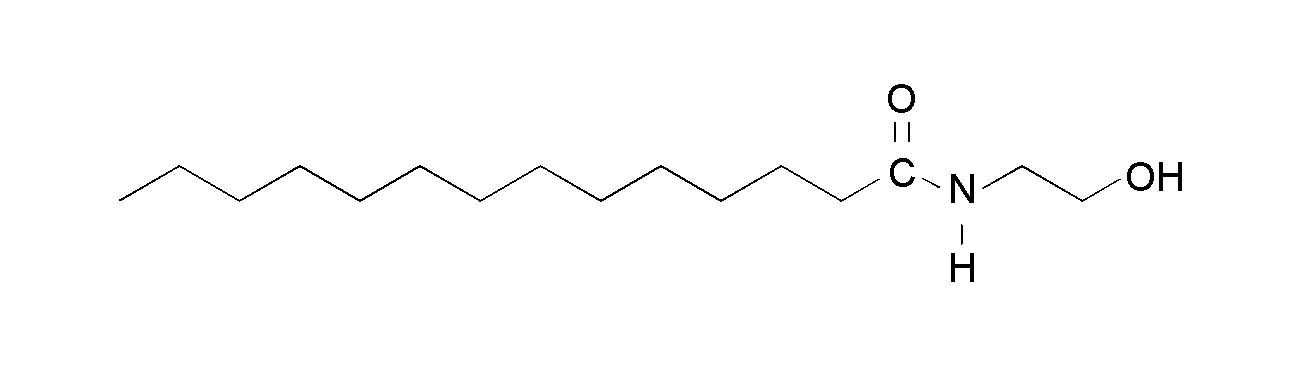
Tetradecanoic acid-(2-hydroxyethyl)amide (N-myristoyl ethanolamine, TDAHA)
3-Hydroxy-N-tridecylpropanoic acid amide, (HTPA)
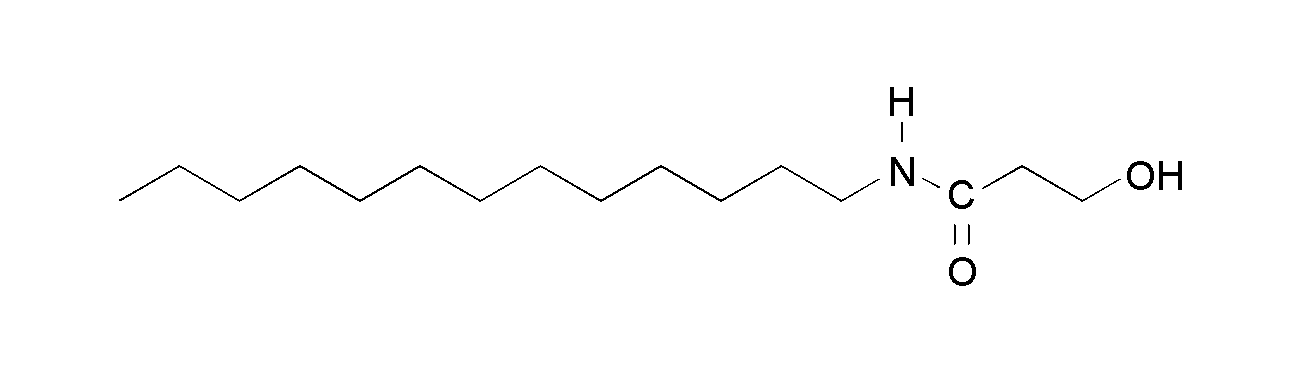
Figure 1. Chemical structures of the N,O-diacyl derivative of ethanolamine, TAOAE (above), and the single-chain amides with exchanged position of the two substituents at the acid amide group N-myristoyl ethanolamine, TDAHA (middle), and 3-hydroxy-N-tridecylpropanoic acid amide, HTPA (bottom).
X-ray Diffraction Measurements
The lattice structure in the condensed monolayers of the 1:1 TAOAE/TDAHA and TAOAE/HTPA mixtures was investigated using grazing incidence X-ray diffraction measurements at the BW1 beamline, HASYLAB, DESY (Hamburg, Germany). At BW1, a Langmuir film balance equipped with Wilhelmy plate was positioned in a hermetically closed container filled with helium for monitoring the lateral pressure. A monochromatic synchrotron X-ray beam ( = 1.304 Å) was adjusted to strike the helium/water interface at a grazing incidence angle αi = 0.85αc (αc = 0.13°) lighting up roughly 250 mm2 of the surface. The trough was laterally moved during the measurements to prevent any sample damage by the strong X-ray beam. For the measurement of the diffracted signal a MYTHEN detector system (PSI, Villigen, Switzerland) was rotated to scan the in-plane Qxy component values of the scattering vector. The vertical strips of the MYTHEN measured the out-of-plane Qz component of the scattering vector between 0.0 and 0.75 Å-1. The diffraction data consisted of Bragg peaks at diagnostic Qxy values. The in-plane lattice repeat distances d of the ordered monolayer structure were calculated from the Bragg peak positions: d=2/Qxy. The in-plane coherence length Lxy, was approximated from the full-width at half maximum (FWHM) of the Bragg peak using Lxy~0.9(2)/FWHM (Qxy). The diffracted intensity normal to the interface was integrated over the Qxy window of the diffraction peak to calculate the corresponding Bragg rod. The thickness of the monolayer was estimated from the FWHM of the Bragg rod using 0.9(2)/FWHM(Qz). Experimental details have been described in the literature [30-33].
Results and Discussion
As demonstrated in recent papers, the exchange of the position of the two substituents at the acid amide group of the single-chain amphiphiles TDAHA and HTPA reveal clear differences in the π-A isotherms of the monolayers.
The inclined plateau region of fluid/condensed phase transition was found for N-myristoyl ethanolamine, TDAHA, between 10°C and 36°C, whereas for 3-hydroxy-N-tridecylpropanoic acid amide, HTPA, between >0°C and 20°C. At low temperatures <10 °C, both amphiphiles show a striking second weak inflection point at A < Ac, indicating the existence of a second phase transition between two condensed phases, which is strongly dependent on temperature for the TDAHA monolayers and nearly independent of temperature for the HTPA monolayers. The area change after the second inflection point is small compared with the main phase transition.
The p-A isotherms of the N,O-diacyl derivative of ethanolamine, TAOAE, are similar to those of the most usual monolayers of amphiphiles and show after a sharp break point (Ac) a nearly horizontal plateau region of the fluid/condensed phase transition.
In the following, the p-A isotherms of 1:1-mixtures of the selected N,O-diacyl derivative of ethanolamine, TAOAE, with the amphiphilic single-chain amides TDAHA and HTPA have been studied at two selected temperatures ( 5 °C and 25 °C) to obtain basic information about its role in mixed systems.
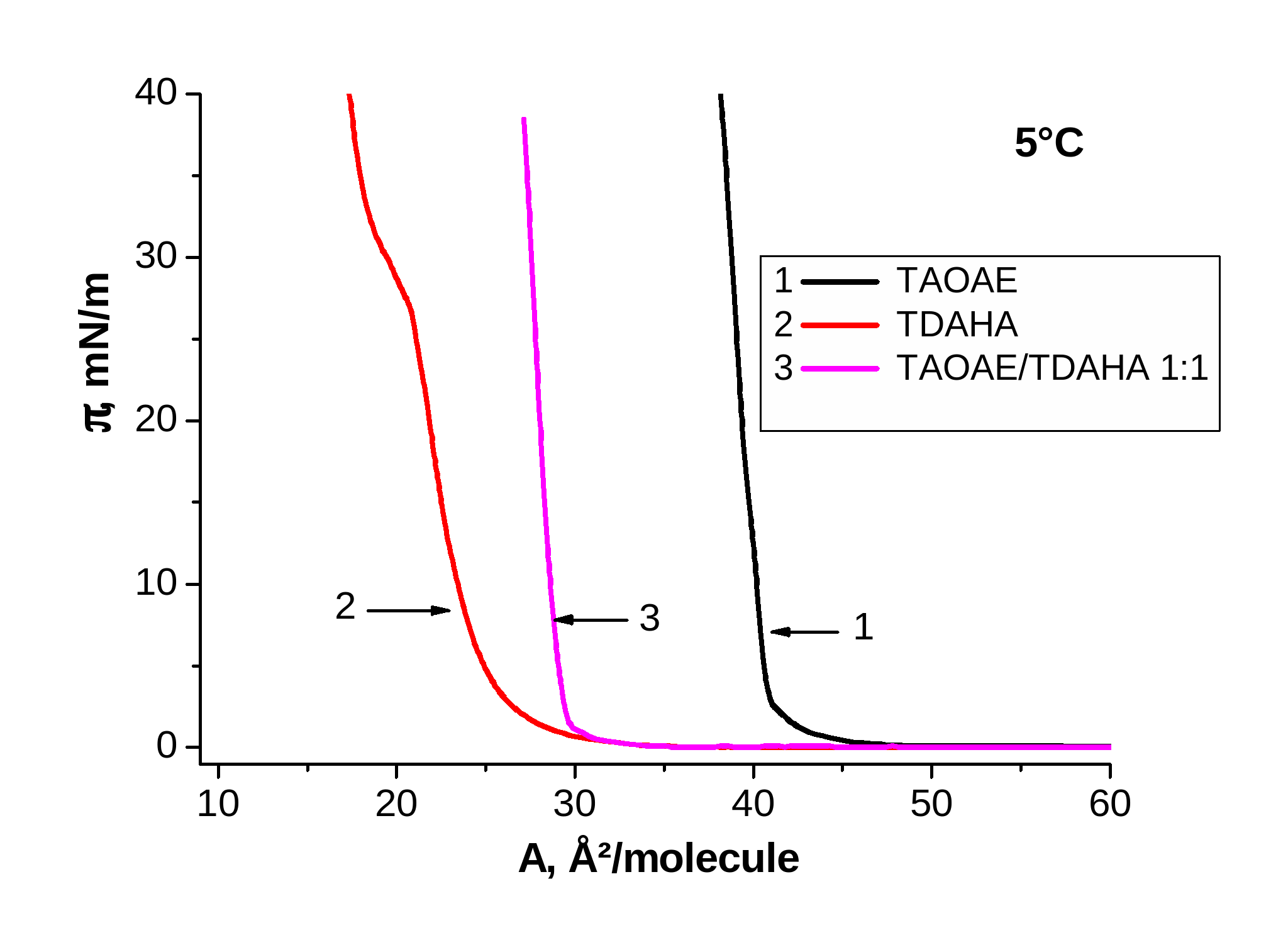
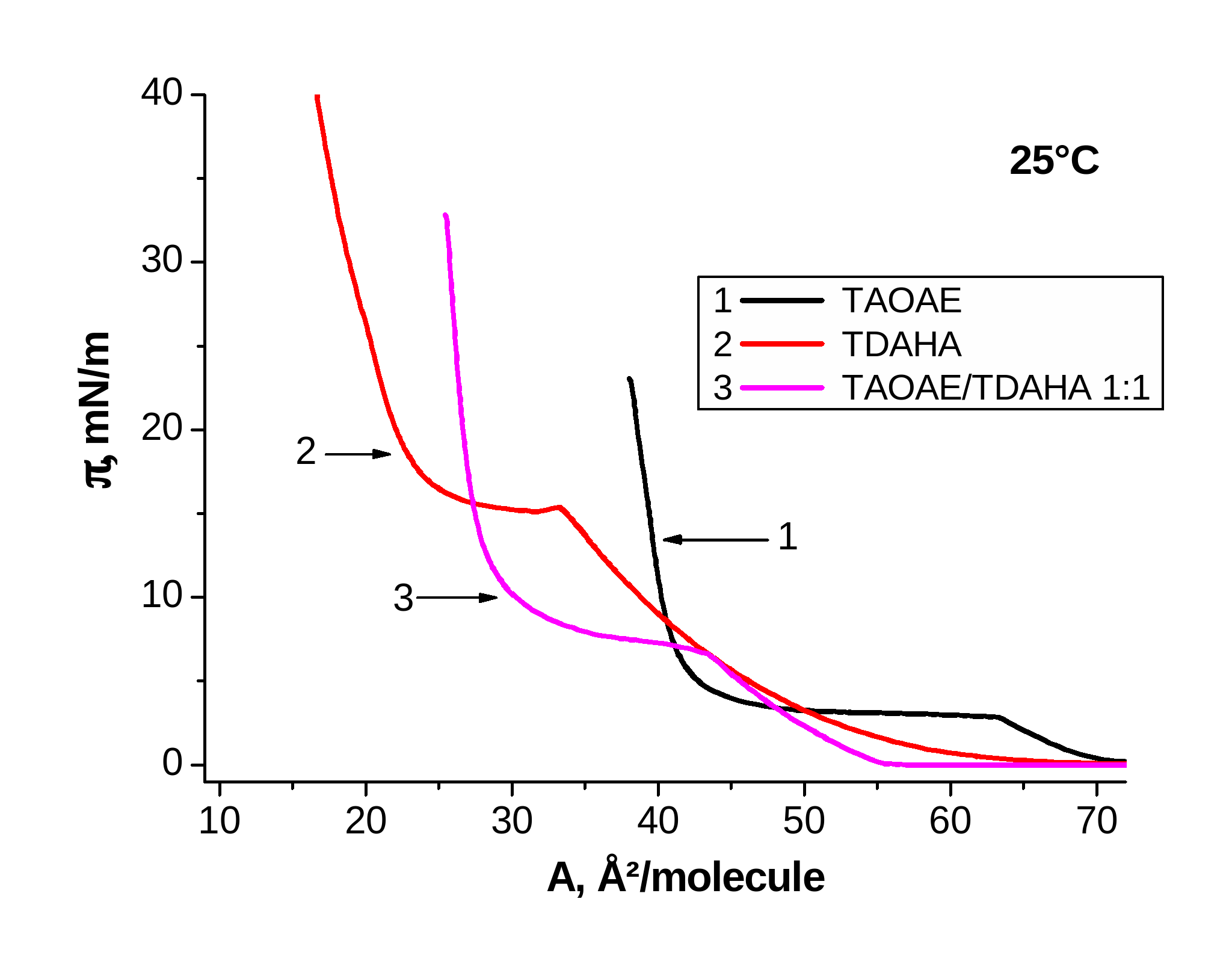
Figure 2. p-A isotherms of TAOAE; TDAHA and 1:1 TAOAE/TDAHA monolayers spread on water and measured at 5 °C and 25 °C
Figure 2 shows the p-A isotherms of the pure double-chain N,O-diacylated ethanolamine TAOAE, the single-chain N-acylated ethanolamine TDAHA and the 1:1 TAOAE/ TDAHA mixtures at 5 °C (left) and 25 °C (right). At 5°C, the main fluid/condensed phase transition of the pure components TAOAE and TDAHA, but also of the 1:1 TAOAE/ TDAHA mixture takes place already at zero pressure. It is interesting to note that the second phase transition between two condensed phases occurring only in the TDAHA monolayer at 5°C cannot be observed in the 1:1 mixed TAOAE/ TDAHA monolayer. At 25 °C, the p-A isotherms of the two single components as well as the their mixture show the well developed characteristics for the first order fluid/condensed phase transition (with the phase transition point at 3.8 mN/m for TAOAE, at 15.4 mN/m for TDAHA and at 7.3 mN/m for the 1:1 TAOAE/ TDAHA mixture). Obviously, the two components of the TAOAE/ TDAHA mixture form a homogeneously mixed system having only one fluid/condensed phase transition whose phase transition pressure is closer to that of the double-chain N,O-diacylated ethanolamine TAOAE.
Figure 3 shows in similar way the p-A isotherms of the pure double-chain N,O-diacylated ethanolamine TAOAE, the single-chain HTPA, which is different from the chemical structure of TDAHA only in the position of the two substituents at the amide group, and the 1:1 TAOAE/HTPA mixtures at 5 °C (left) and 25 °C (right). In this case, the temperature dependence of the p-A isotherms of HTPA are considerably different from that of TDAHA. At 5°C, the HTPA monolayer has the fluid/condensed phase transition pressure at 11.4 mN/m and a weak inflection point for the second phase transition between two condensed phases at 18 mN/m. Similar to pure TAOAE, the fluid/condensed phase transition of the 1:1 TAOAE/HTPA mixture takes place already at zero pressure but the steep pressure increase is shifted to smaller area values and a second condensed/condensed phase transition does not occur. The comparison of the p-A isotherms measured at 25 °C reveals the large difference in the fluid/condensed phase transition pressure which is at 3.8 mN/m for TAOAE and already in the region of the irreversible collapse at about 39 mN/m for HTPA. It is obvious that also the 1:1 mixture shows the characteristics of a homogeneously mixed system with the fluid/condensed phase transition pressure of 8.3 mN/m, which is considerably closer to that of TAOAE than to HTPA.
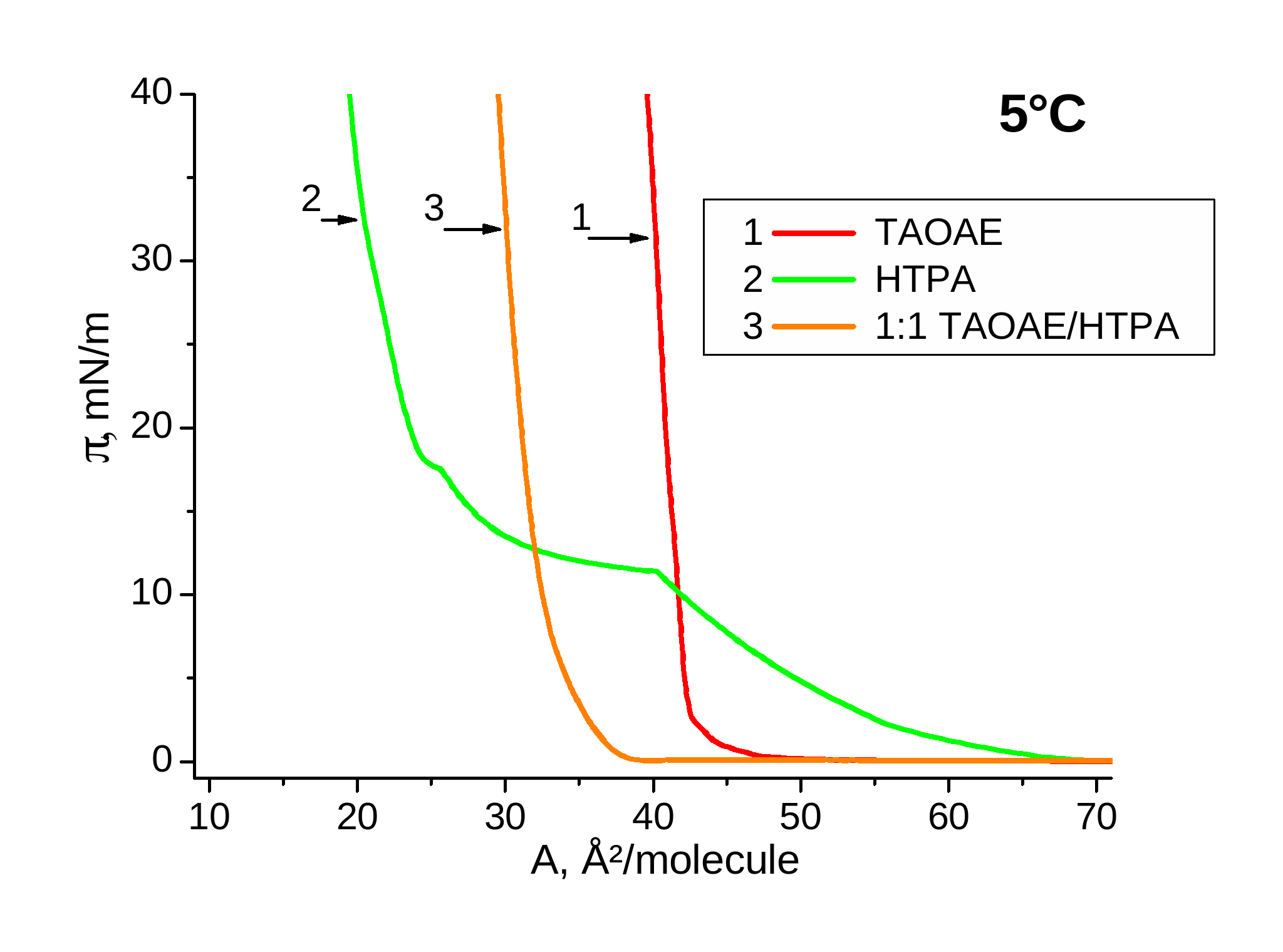
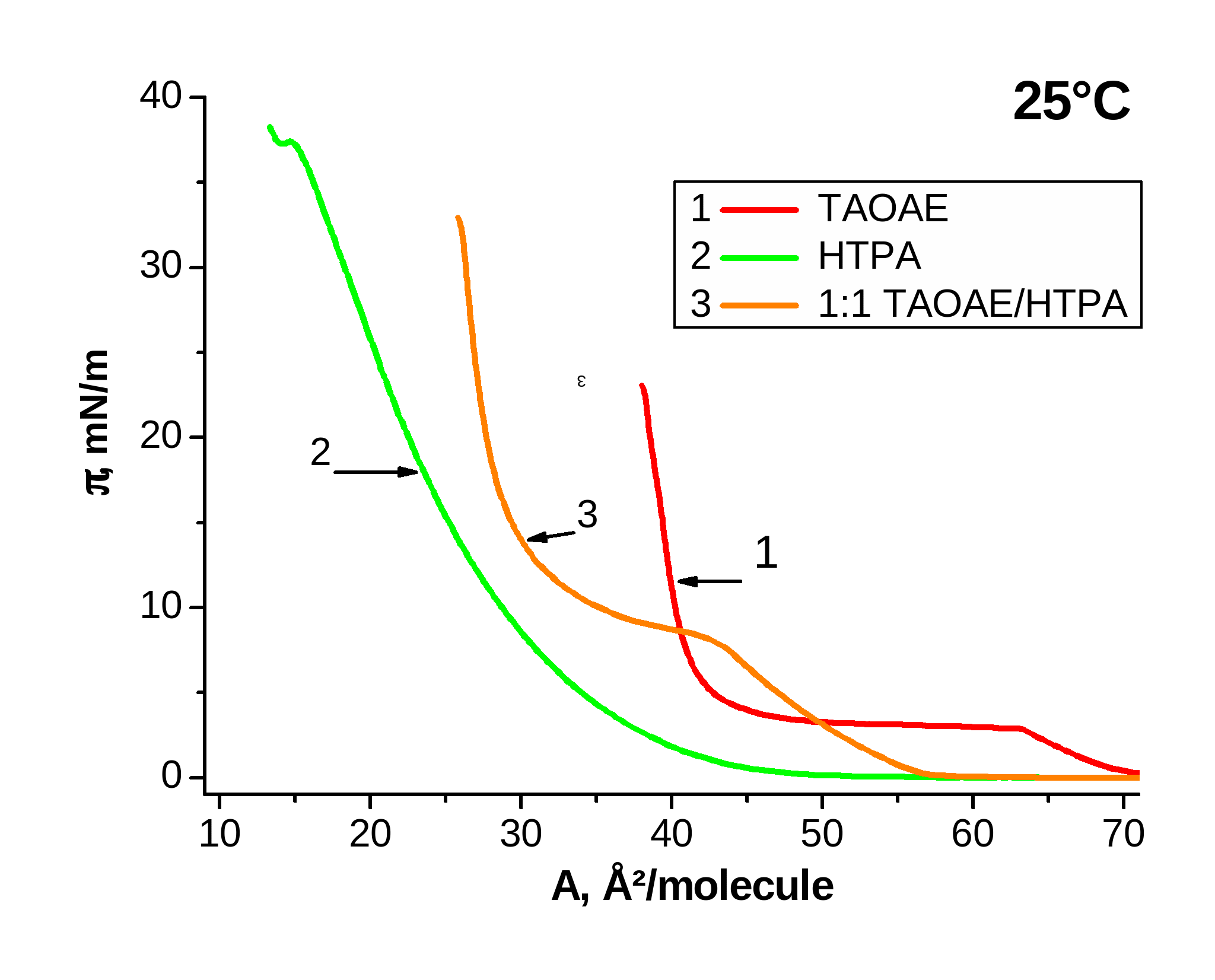
Figure 3. p-A isotherms of TAOAE; HTPA and 1:1 TAOAE/HTPA monolayers spread on water and measured at 5 °C and 25 °C
In the case of pure HTPA (Figure 3, right, curve 2) and pure TDAHA (Figure 2, right, curve 2), the isotherms at higher temperatures are shifted to unusual small area values and also the slope is reduced which is caused by their enhanced solubility at 25 °C and consequently, by desorption of a certain amount of the monolayer material during compression.
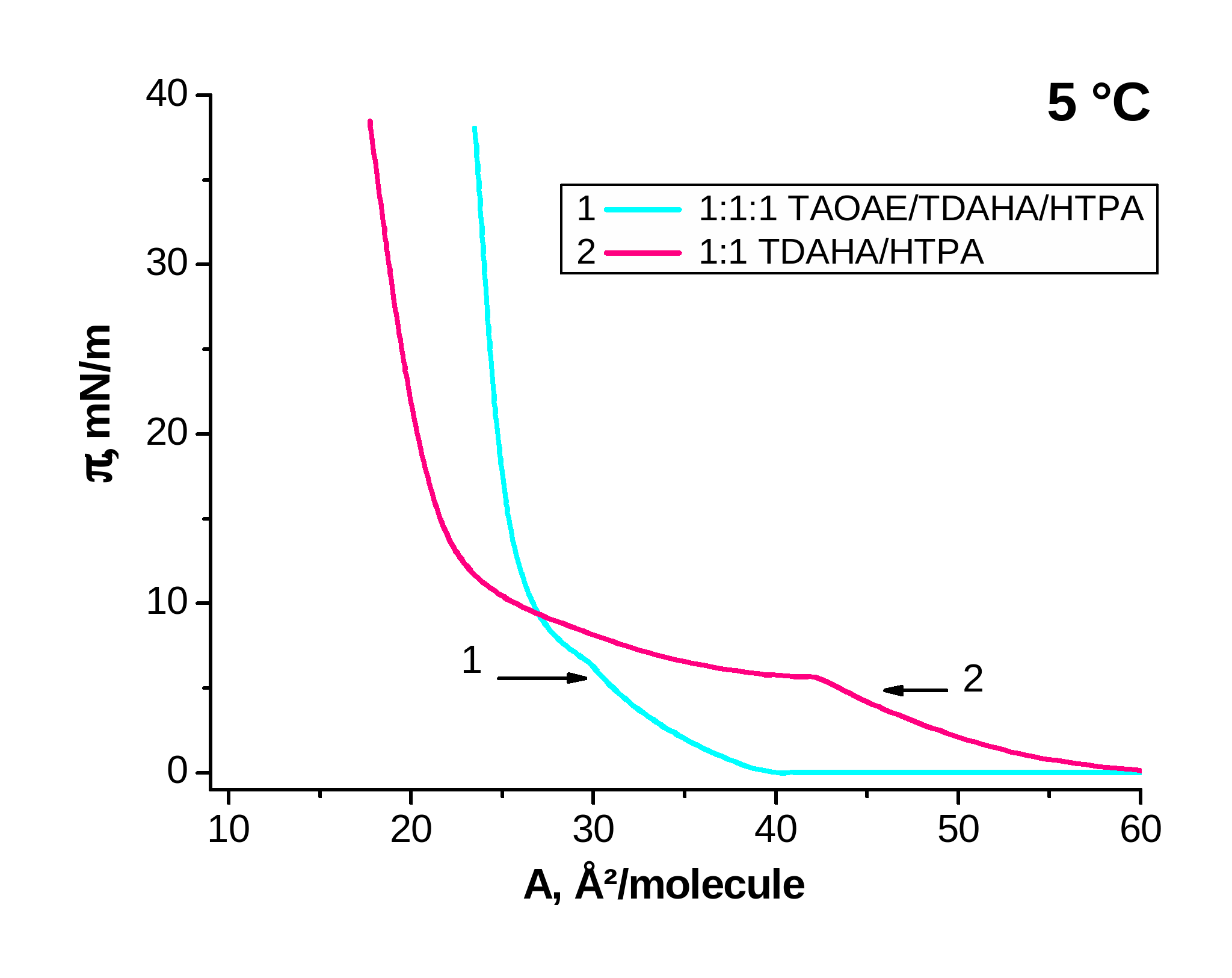
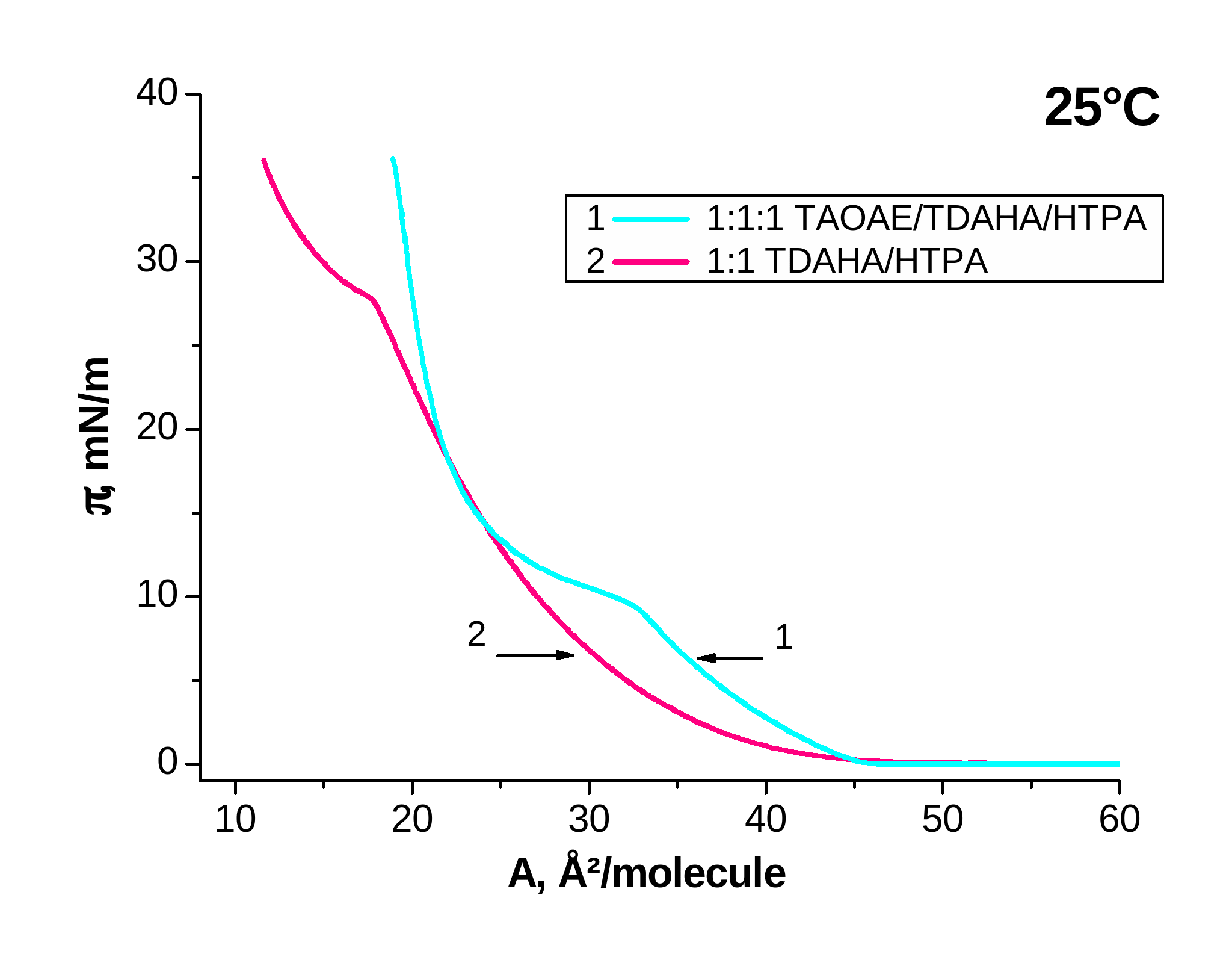
Figure 4. p-A isotherms of mixed 1:1 TDAHA/HTPA and 1:1:! TAOAE/TDAHA/HTPA monolayers spread on water and measured at 5 °C and 25 °C
Now Figure 4 demonstrates the effect of the double-chain TAOAE on the mixture of the single-chain TDAHA/HTPA by comparison of 1:1 TDAHA/HTPA with 1:1:1 TAOAE/TDAHA/HTPA at 5 °C (Figure 4, left) and 25 °C (Figure 4, right). The mixed 1:1 TDAHA/HTPA monolayer forms a homogeneously mixed system indicated by one fluid/condensed phase transition with the usual temperature dependent shift of the transition pressure (5.7 mN/m at 5 °C and 27.7 mN/m at 25 °C; see Figure 4). The second condensed/condensed phase transition occurring in the two single components at low temperatures (5°C) is missing in the 1:1 and 1:1:1 mixtures. Also the three-component 1:1:1 TAOAE/TDAHA/HTPA monolayer forms a homogeneously mixed system with only one fluid/condensed phase transition with a transition pressure of 9.7 mN/m at 25 °C, whereas it is already at zero pressure at 5 °C.
Summarizing, it results from the p-A isotherm studies of the 1:1 and 1:1:1 mixtures with the double-chain N,O-diacylated ethanolamine, TAOAE, that always a homogeneously mixed system is formed indicated by one fluid/condensed phase transition with the usual temperature dependent shift of the transition pressure. The phase transition pressure in the mixed sytems is always closer to that of the double-chain N,O-diacylated ethanolamine TAOAE. At low temperatures (5 °C), the second phase transition between two condensed phases, as observed for the pure single-chain amphiphiles, does not occur.
Information about the lattice structure of the condensed phase of the mixed monolayers can be obtained using grazing incidence X-ray diffraction (GIXD). The two-dimensional structure of the condensed monolayers of the three single components has been determined and discussed in recent papers, for the single-chain N-acylated ethanolamine TDAHA in ref. [23], for the single-chain HTPA in ref. [18] and for double-chain N,O-diacylated ethanolamine TAOAE in ref. [22].
As presented in ref. [23], the monolayer of the single-chain N-acylated ethanolamine TDAHA shows three Bragg peaks at all pressures between the first and the second phase transition indicating an oblique lattice with strongly tilted alkyl chains in this region. Above the second transition pressure, the condensed monolayer phase changes to an orthorhombic structure (two Bragg peaks) with NNN (next nearest neighbors) tilted chains. The lattice data of two representative pressures for the two phases of the TDAHA monolayer measured at 5 °C are listed in Table 1 [23]. It is seen that the cross-sectional area, A0, amounts to 19.3 Å2 in the oblique phase and decreases to 18.8 Å2.in the orthorhombic phase.
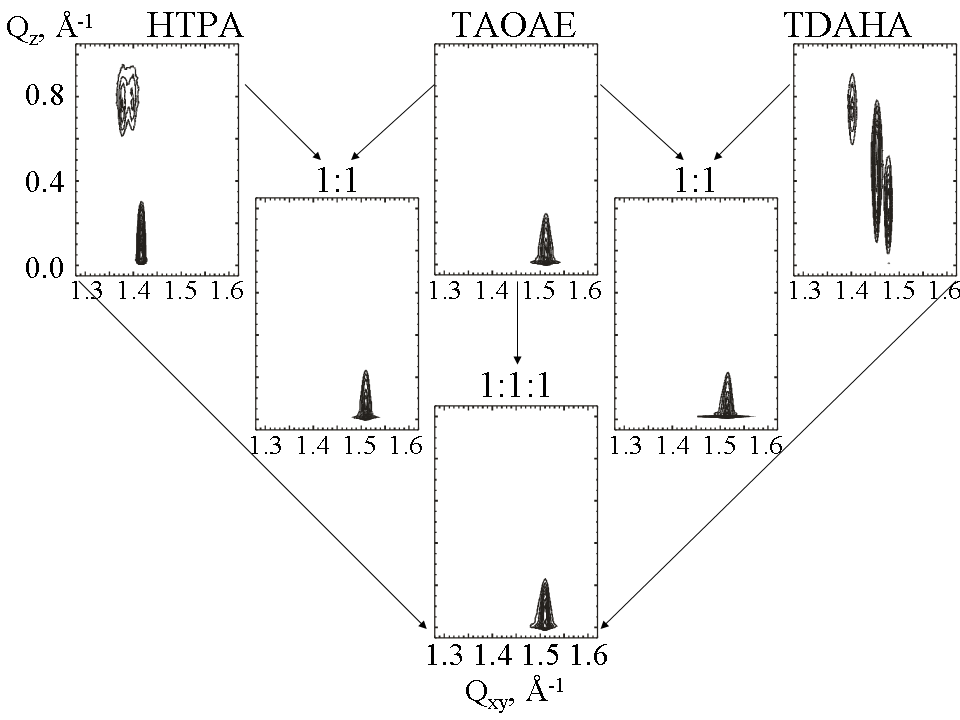
Figure 5. Grazing incidence X-ray diffraction data for TAOAE, HTPA and TDAHA monolayers and the corresponding binary (1:1) and ternary (1:1:1) mixtures spread on water at 5 °C and compressed to 10 mNm-1. The diffracted intensity, corrected for polarization, effective area, and Lorentz factor, is plotted as contour lines of equal intensity versus the in-plane component Qxy and the out-of-plane component Qz of the scattering vector (the same Qxy and Qz scale is used in all cases). The structure in the binary as well as ternary mixtures is dominated by the double-chain TAOAE.
Table 1. Lattice parameters a, b, c and α, β, γ of the unit cell, lattice distortion, chain tilt t from the surface normal, in-plane area Axy per chain, cross-sectional area A0 of TDAHA monolayers at 5 °C and different lateral pressures π
|
π (mN/m) |
a (Ǻ) b (Ǻ) c (Ǻ) |
α (°) β (°) γ (°) |
distortion |
t (°) |
Axy (Ǻ2) |
A0 (Ǻ2) |
|
10 |
4.891 5.037 5.120 |
122.4 119.6 117.9 |
0.05310 |
27.8 |
21.8 |
19.3 |
|
30 |
4.887 4.580 4.580 |
115.5 122.2 122.2 |
0.08839 |
7.4 |
18.9 |
18.8 |
The other single-chain acid amide amphiphile HTPA shows also three Bragg peaks between the first and the second phase transition at low temperatures. The characteristics of the three Bragg peaks change abruptly at the second transition pressure. That means, the two condensed phases are oblique but having completely different characteristics. The observed structure at lateral pressures below the transition pressure is quite similar to that of a rectangular unit cell with NN-tilted alkyl chains. Its tilt angle decreases more strongly with increasing pressure (0.47°/mN·m-1) and the cross-sectional area is rather small (19.5 Å2). The lattice data of two representative pressures for the two oblique phases of the HTPA monolayer measured at 3 °C are listed in Table 2 [18].
Table 2. Lattice parameters a, b, c and α, β, γ of the unit cell, lattice distortion, chain tilt t from the surface normal, in-plane area Axy per chain, cross-sectional area A0 of HTPA monolayers at 3 °C and different lateral pressures π
|
π (mN/m) |
a (Ǻ) b (Ǻ) c (Ǻ) |
α (°) β (°) γ (°) |
distortion |
t (°) |
Axy (Ǻ2) |
A0 (Ǻ2) |
|
12 |
5.123 5.202 5.265 |
121.4 119.9 118.7 |
0.0314 |
33.7 |
23.4 |
19.5 |
|
30 |
4.818 4.888 4.994 |
121.6 120.3 118.1 |
0.0417 |
15.9 |
20.8 |
20.0 |
The 2D lattice structure of the double-chain N,O-diacyl substituted ethanolamine TAOAE shows clear differences compared to the acid amide amphiphiles TDAHA and HTPA which have only one alkyl chain. This is clearly demonstrated by the presentation as contour plots of equal intensity versus the in-plane component Qxy and the out-of-plane component Qz of the scattering vector, measured at 5 °C and 10 mN/m and using the same Qxy and Qz scale in all cases (see Figure 5, above). At all pressures, only one diffraction peak was observed, indicating hexagonal packing of the TAOAE molecules oriented perpendicular to the surface in a LS phase. No second phase transition within the condensed phase region exists. For the selected lateral pressures of 4 and 10 mN/m at 5°C, the Bragg peak positions, the lattice parameters, the in-plane correlation lengths and monolayer thicknesses are listed in Table 3. The area of the unit cell, Axy = A0, decreases very slightly from 20.0 Ų at 4 mN/m to 19.9 Ų at 10mN/m indicate a certain mobility of the alkyl chains in a rotator phase.
Table 3. Lattice Parameters a, b, c and , , γ of the Unit Cell; Chain Tilt, t, from the Surface Normal; in-plane Area, Axy, per Chain; and Cross-Sectional Area, A0; in plane Correlation Length and Thickness of TAOAE monolayers at 5 °C
|
mN/m |
peak position Qxy, Å-1 Qz, Å-1 |
a=b=c Å |
== deg |
Axy = A0 Ų |
tilt deg |
correlation length, Å |
thickness Å |
|
4 |
1.510 0 |
4.805 |
120 |
20.0 |
0 |
378 |
20.8 |
|
10 |
1.512 0 |
4.798 |
120 |
19.9 |
0 |
333 |
20.2 |
The monolayers formed by the 1:1 mixtures of TAOAE with TDAHA or HTPA exhibit also only one diffraction peak at the same position for the TAOAE:TDAHA mixture and at a very slightly lower position for the TAOAE:HTPA mixture (Figure 5, middle), indicating hexagonal packing of non-tilted molecules in the LS phase. The same contour plot as for the pure TAOAE monolayers was also found for the three-component 1:1:1 TAOAE:TDAHA:HTPA mixture (Figure 5, bottom). The identical contour plots of the binary and ternary mixtures to that of the pure TAOAE monolayers provide an unequivocal evidence that always a homogeneously mixed system is formed completely dominated by the lattice structure of the double-chain N,O-diacylated ethanolamine, TAOAE. This is demonstrated for selected lateral pressures at 5°C by the Bragg peak positions, the lattice parameters, the in-plane correlation lengths and monolayer thicknesses listed in Table 4 for 1:1 TAOAE:TDAHA monolayers, in Table 5 for 1:1 TAOAE:HTPA monolayers and in Table 6 for 1:1:1 TAOAE-TDAHA-HTPA monolayers.
Table 4. Lattice Parameters a, b, c and , β, γ of the Unit Cell; Chain Tilt, t, from the Surface Normal; in-plane Area, Axy, per Chain; and Cross-Sectional Area, A0; in plane Correlation Length and Thickness of mixed 1:1 TAOAE:TDAHA monolayer at 5 °C
|
mN/m |
Qxy
|
Qz Å-1 |
a=b=c Å |
=β=γ deg |
Axy = A0 Ų |
tilt deg |
correlation length, Å |
thickness Å |
|
1 |
1.511 |
0 |
4.802 |
120 |
20.0 |
0 |
345 |
18.4 |
|
10 |
1.515 |
0 |
4.789 |
120 |
19.9 |
0 |
345 |
19.7 |
Table 5. Lattice Parameters a, b, c and , β, γ of the Unit Cell; Chain Tilt, t, from the Surface Normal; in-plane Area, Axy, per Chain; and Cross-Sectional Area, A0; in plane Correlation Length and Thickness of mixed 1:1 TAOAE:HTPA monolayers at 5 °C
|
mN/m |
peak position Qxy, Å-1 Qz, Å-1 |
a=b=c Å |
=β=γ |
Axy = A0 Ų |
tilt deg |
correlation length, Å |
thickness Å |
|
4 |
1.507 0 |
4.814 |
120 |
20.1 |
0 |
395 |
18.2 |
|
10 |
1.510 0 |
4.805 |
120 |
20.0 |
0 |
425 |
19.5 |
Table 6. Lattice Parameters a, b, c and , β, γ of the Unit Cell; Chain Tilt, t, from the Surface Normal; in-plane Area, Axy, per Chain; and Cross-Sectional Area, A0; in plane Correlation Length and Thickness of mixed 1:1:1 TAOAE-TDAHA-HTPA monolayer at 5 °C
|
mN/m |
Qxy Å-1 |
Qz Å-1 |
a=b=c Å |
=β=γ deg |
Axy = A0 Ų |
tilt deg |
correlation length, Å |
thickness |
|
4 |
1.506 |
0 |
4.818 |
120 |
20.1 |
0 |
377 |
19.7 |
|
10 |
1.510 |
0 |
4.805 |
120 |
20.0 |
0 |
332 |
19.7 |
The dominant effect of the double-chain N,O-diacylated ethanolamine TAOAE on the morphology of the mesoscopic condensed phase domains of the studied binary and ternary mixed monolayers can be visualized by BAM studies. Previous studies of various amphiphilic acid amides have shown that generally a dendritic domain shape is realized.
TDAHA




5 °C 15 °C 15 °C 25 °C
HTPA




5 °C 10 °C 10 °C 15 °C
TAOAE



25 °C growth kinetics
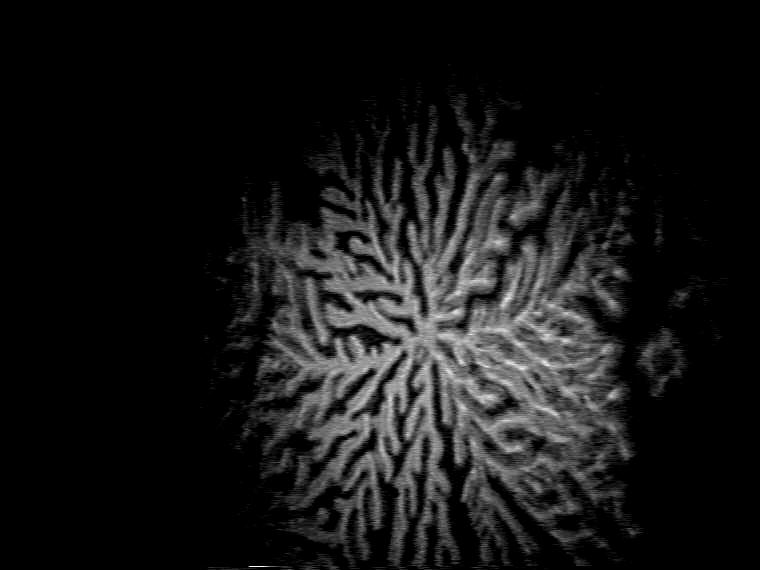
Figure 6. Characteristic domain shapes of TDAHA (top) and HTPA (middle)) monolayers at selected temperatures and domain growth of TAOAE monolayer at 25 °C (bottom.
However, specific discrimination between the pure amphiphilic acid amides is possible. This is demonstrated by the domain morphology of the studied three compounds observed at different temperatures (Figure 6). General differences in the shape are seen between the single-chain acid amide amphiphiles TDAHA and HTPA and the double-chain N,O-diacyl substituted ethanolamine TAOAE. The domains of the single-chain acid amide amphiphiles have higher crystallinity. Correspondingly, they grow with straight main axes, and the main axes develop numerous straight branches on both sides in the succeeding growth stages, clearly indicating the dendritic shape. HTPA is characterized by particularly high crystallinity, six straight main axes grow from a centre in a regular distance of about 60 degrees from each other. The TDAHA dendrites tend to form large domain and are at higher temperature (25 °C) less crystalline.
The differences in the domain shape of the TAOAE monolayers can be clearly seen from the growth kinetics presented in Figure 6, bottom, for 25 °C, but is very similar at other temperatures. Bent arms growing from the compact centre in all directions indicate the fractal nature of the domain shape. Tip splitting under the formation of doubloons and often conspicuous thickening at the tip become obvious in the further stages of the domain growth.
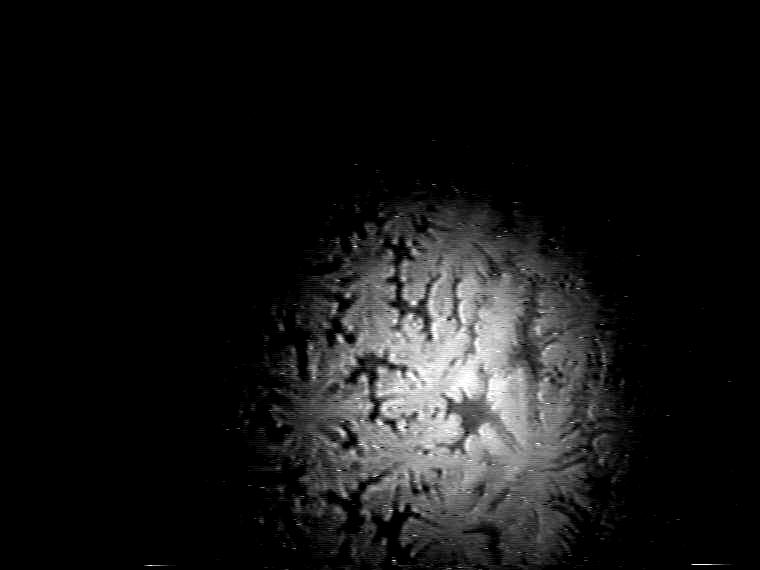
The dominant effect of the double-chain TAOAE on the morphology of the mesoscopic condensed phase domains in the binary 1:1 TAOAE- TDAHA (Figure 7) and TAOAE- HTPA (Figure 8) monolayers can be clearly seen. According to the selected temperature for the -A isotherms (see Figures 2 and 3), the domain growth is presented for 5 °C and 25 °C. However, at the low temperature of 5 °C the main fluid/condensed phase transition occurs already at zero pressure in both binary systems so that the conditions of nucleation and growth for the formation of regular condensed phase structures are not given. Rather irregular structures are formed depending on the spreading conditions. Therefore, for the realisation of reasonable conditions for the development of the condensed phase domains on the basis of nucleation and growth from the gaseous phase, the monolayers were spread at 60 Ų/molecule and 25 °C, and then cooled to 5 °C. Figure 7 shows the domain development in the plateau region of the -A isotherm (see Figure 2) by compression at 25 °C and during cooling to 5 °C at the constant area of 60 Ų/molecule. Despite some differences caused by the different kinetic conditions at the two temperatures the general shape characteristics agree with each other. The comparison with the domain shapes of the single components shows a clear resemblance to those of TAOAE. A similar situation exists in the case of the binary 1:1 TAOAE-HTPA monolayer. Figure 8 shows the domain shapes observed under conditions comparable to those of the TAOAE- TDAHA monolayers at 25 °C and 5 °C.
1:1 TAOAE-TDAHA



Growth kinetics at 25°C

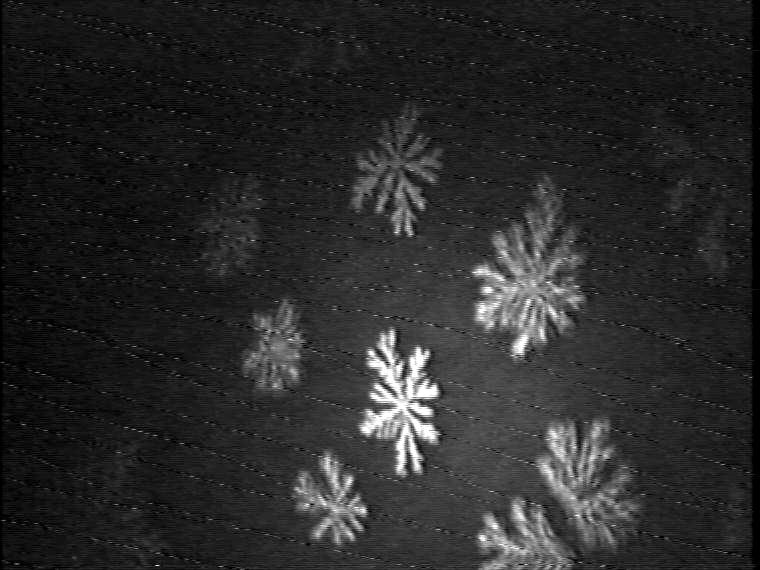


Growth kinetics at cooling from 25°C to 5°C at 60 Ų/molecule
Figure 7. Development of the domain growth in binary 1:1 TAOAE-TDAHA monolayers.
Top: Domain growth in the plateau region of the -A isotherm (see Figure 2) during compression at 25 °C. Bottom: Domain growth during cooling from 25°C to 5°C at 60 Ų/molecule.
Also the domain shapes of the TAOAE-HTPA monolayers resemble those of the pure TAOAE monolayers. That means, also the mesoscopic domain structures indicate the dominance of the double-chain TAOAE in mixed monolayers with single-chain acid amide amphiphiles.
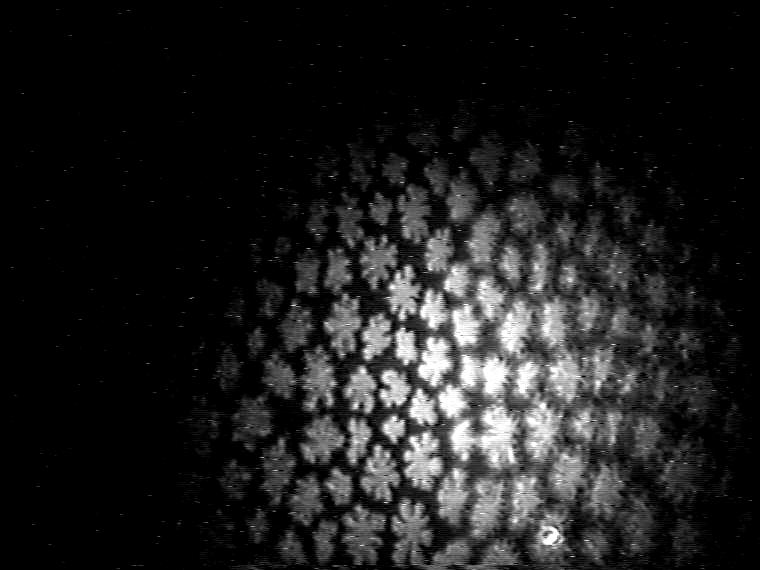
Finally, the morphological characteristics of the mesoscopic domain shapes of the ternary 1:1:1 TAOAE-TDAHA-HTPA monolayers show that also this system is dominated by the double-chain TAOAE amphiphile (Figure 9). Despite the high proportion of the single-chain acid amide amphiphiles TDAHA and HTPA there is no indication of crystalline dendrites with straight axes. Rather bent arms and tip splitting under the formation of doubloons indicate the fractal nature of the domain shape as the main characteristics of the pure TAOAE domains.
As a consequence, it can be concluded that the presence of the double-chain N,O-diacylated ethanolamine TAOAE as amphiphilic impurity both in the single-chain TDAHA and its mixture with other amphiphilic single-chain acid amides dominates the most important characteristics of single-chain acid amide monolayers in mesoscopic and molecular scales. The probability for the presence of N,O-diacylated ethanolamine as impurity in N-acylated ethanolamine (e.g. TDAHA) is high because the amino and hydroxy groups of ethanolamine offer the possibility for N- and O-acylation [20,21].
1:1 TAOAE-HTPA, 25°C



1:1 TAOAE-HTPA, 5°C
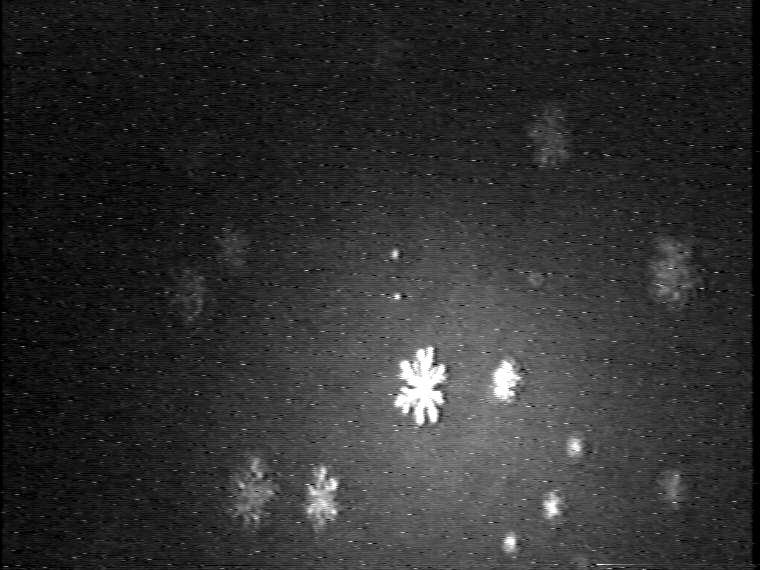
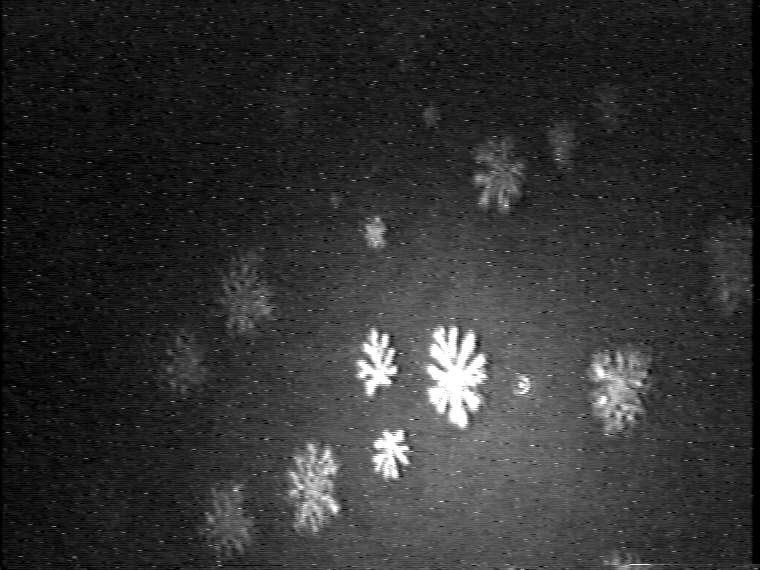
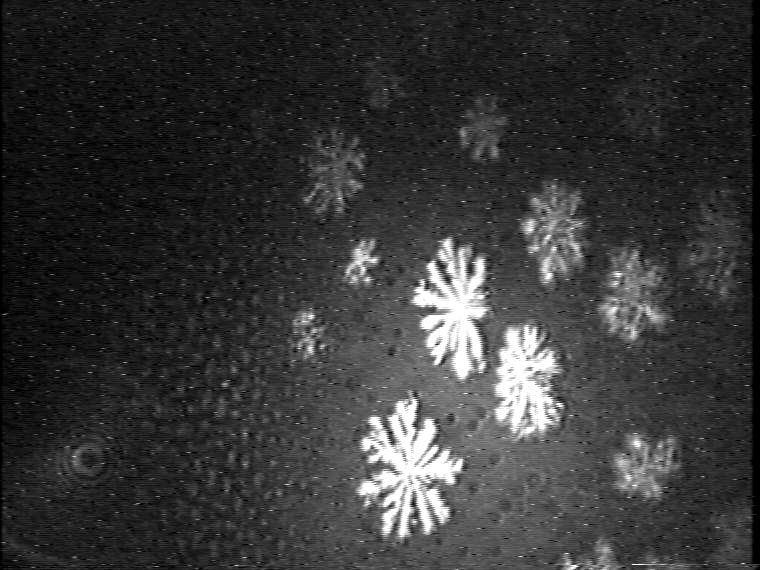
Figure 8. Development of the domain growth in binary 1:1 TAOAE-HTPA monolayers. Top: Domain growth in the plateau region of the -A isotherm (see Figure 2) during compression at 25 °C. Bottom: Domain growth during cooling from 25°C to 5°C at 60 Ų/molecule.
1:1:1 TAOAE-TDAHA-HTPA
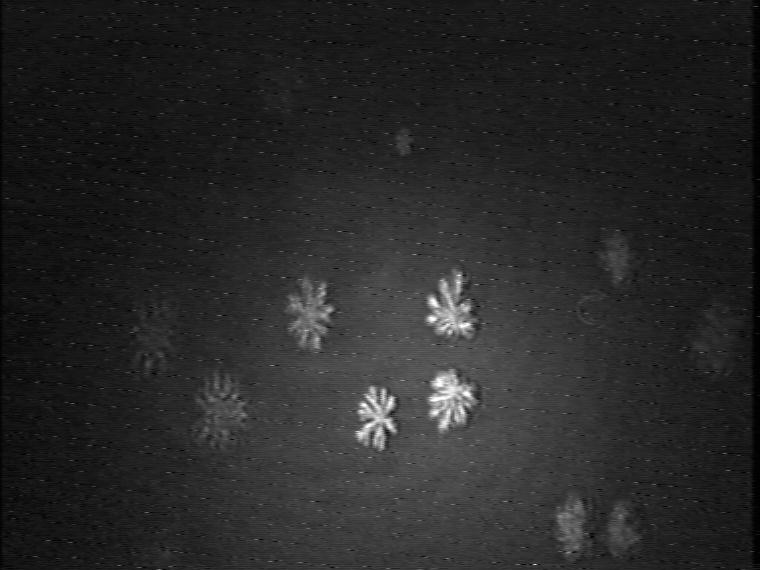
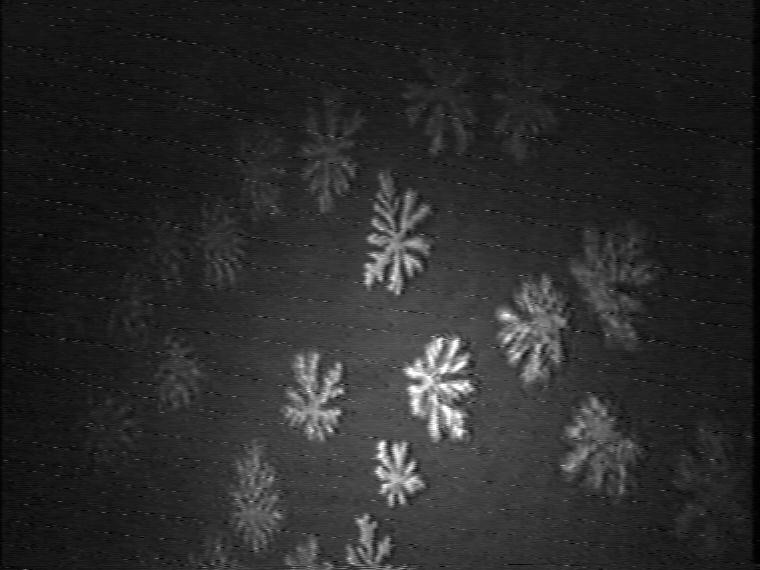


Figure 9. Development of the domain growth in ternary 1:1:1 TAOAE-TDAHA-HTPA monolayers after cooling from 25°C to 5°C at 60 Ų/molecule.
Conclusions
Fundamental differences exist in the main characteristics of Langmuir monolayers, on the one hand, of the single-chain acid amide amphiphiles, demonstrated by the N-acylated ethanolamine TDAHA and by HTPA whose chemical structure differs only in the position of the two substituents at the amide group, and on the other hand, of the double-chain N,O-diacylated ethanolamine TAOAE. This can be concluded by the comparison of surface pressure – area (p-A) isotherms, the mesoscopic morphology, and the two-dimensional lattice structure of the condensed phases. The same characteristics have been studied in the binary mixtures TDAHA/TAOAE, HTPA/TDAHA, HTPA/TAOAE (1:1) and ternary mixture TAOAE/TDAHA/HTPA (1:1:1) at low (5 °C) and usual (25 °C) temperatures to allow the comparison with the single components. The results obtained on the basis of the three methods indicate clearly that all binary 1:1 monolayers and the ternary 1:1:1 monolayer form homogeneously mixed systems. The comparison of the characteristics of the GIXD and BAM measurements provides an unequivocal evidence for the dominance of the double-chain TAOAE in the binary and ternary monolayers mixed with single-chain acid amide amphiphiles TDAHA and HTPA in mesoscopic and molecular scales. Governed by the conditions of the synthesis the probability for the presence of N,O-diacylated ethanolamine, such as TAOAE, as impurity in N-acylated ethanolamine (e.g. TDAHA) is high so that in such systems the most important characteristics are dominated by the impurity.
Acknowledgement
We thank HASYLAB at DESY, Hamburg, Germany, for beamtime and support.
References
H.M. McConnell, Annu. Rev. Phys. Chem. 42 (1991) 171.
D. Vollhardt, Adv. Colloid Interface Sci. 64 (1996) 143.
I. Kuzmenko, H. Rapaport, K. Kjaer, J. Als-Nielsen, I. Weissbuch, M. Lahav, L. Leiserowitz, Chem. Rev. 101 (2001) 1659.
N. Nandi, D Vollhardt, Chem. Rev. 103 (2003) 4033.
K. Thirumoorthy, N. Nandi, D. Vollhardt, J. Phys. Chem. B 109 (2005) 10820.
G. Weidemann,; D. Vollhardt, Biophys. J. 70 (1996) 2758.
D. Vollhardt, S. Siegel, D.A.Cadenhead, Langmuir 20 (2004) 7670.
D. Vollhardt, S. Siegel, D. Cadenhead, J. Phys. Chem. B 108 (2004) 17448.
J.M. Berg, J.L. Tymoczko, L. Stryer, Biochemistry, 5th ed.; W. H. Freeman & Co.: New York, 2003, p 324.
D.L. Nelson, M. M.Cox, Lehninger, Principles of Biochemistry, 4th ed.;W. H. Freeman & Co.: New York, 2004, p 353.
H. Hühnerfuss, V. Neumann, K.J. Stine, Langmuir 12 (1996) 256.
H.H.O. Schmid, P.C. Schmid, V. Natarajan, Prog. Lipid Res. 29 (1990) 1.
H.S.Hansen, B. Moesgaard, H.H. Hansen, G. Petersen, Chem. Phys. Lipids 108 (2000) 135.
J. Hildreth, Biochem. J. 207 (1982) 363.
D.B.Sarney, E.N. Vulfson, Tibtech 13 (1995) 164.
M. Infante, J. Molinero, P. Bosch, M.R. Julia, P. Erra, J. Am. Oil Chem. Soc. 66 (1989) 1835.
S.Y. Mhaskar, G. Lakshminaratana, J. Am. Oil Chem. Soc. 69 (1992) 643.
G. Brezesinski, C. Stefaniu, S. Nandy, D. Dutta Banik, N. Nandi, D. Vollhardt, J. Phys. Chem. C 2010, 114, 15695.
D. Vollhardt, R. Wagner, J. Phys. Chem. B 110 (2006) 14881.
T. Furutani, H. Ooshima, J. Kato, Enzyme and Microbial Technology 19 (1996) 578.
M. Fernandez-Perez, C. Otero, Enzyme and Microbial Technology 28 (2001) 527.
G. Brezesinski, B. Dobner, C. Stefaniu, D. Vollhardt, Langmuir 27 (2011) 5386.
G. Brezesinski, B. Dobner, C. Stefaniu, D. Vollhardt, J. Phys. Chem. C, DOI: 10.1021/jp200847c.
D. Vollhardt, G. Emrich, Colloids Surf. A 161 (2000) 173.
D. Vollhardt, G. Brezesinski, S. Siegel, G. Emrich, J. Phys. Chem. B 105 (2001) 12061.
T.D. Andreeva, J.G. Petrov, G. Brezesinski, H. Möhwald, Langmuir 24 (2008) 8001.
Hönig. D. Möbius, D. J. Phys. Chem. 95 (1991) 4590.
S. Henon, J. Meunier, Rev. Sci. Instrum. 62 (1991) 936.
D. Vollhardt, Adv. Colloid Interface Sci. 64 (1996) 143.
V.M. Kaganer, I.R. Peterson, R. M. Kenn, M. C. Shih, M. Durbin, P. Dutta, J. Chem. Phys. 102 (1995) 9412.
J. Als-Nielsen, D. Jaquemain, K. Kjaer, M. Lahav, F. Levellier, L. Leiserowitz, Phys. Rep. 246 (1994) 251.
K. Kjaer, Physica B 1994 (198) 100.
V. M. Kaganer, H. Möhwald, P. Dutta, Rev. Mod. Phys. 71 (1999) 779.
Tags: amphiphilic acid, other amphiphilic, nodiacylated, amphiphilic, ethanolamine, longchain, mixed, dominance
- GUIA DE EJERCICIOS DINAMICATRABAJO Y ENERGIA DINAMICA EJEMPLO 1
- THE GRAPHIC SHOWS THE TEXT OF AN ACT PASSED
- FORM 12 WARRANT OF FITNESS SECTION 108 BUILDING ACT
- SESIÓN CLÍNICA DIAPOSITIVA DE LA 1 A LA 13
- SUSAN MOSE & OLE RAVN DIGTBOGEN RYTMEN OG DIGTETS
- ORGANIGRAMA INSTITUCIONAL DIRECCIÓN INSPECTORÍA GENERAL UNIDAD TÉCNICO
- AERONÁUTICA CIVIL INFORME EMPRESAS AÉREAS DEL TRANSPORTE AÉREO
- LESEN SACHTEXTE |NAME |FARBEDATUM ☐ ☐ ☐ NACHSCHAUEN UND
- PLACE OF TECHNICAL SUPPORT ORGANIZATIONS IN NUCLEAR ENERGY DEVELOPMENT
- KERTAS KERJA PROJEK PERIKANAN LAUT DALAM (ZON C2)
- TANMENET OSZTÁLYFŐNÖKI EGÉSZSÉGNEVELÉS 4 OSZTÁLY 20092010 TANÉV HETI ÓRASZÁM
- 33 NASLOV RADA NA HRVATSKOM JEZIKU SIMPOZIJ DOKTORSKOG STUDIJA
- SANTO TOMÁS SUMA TEOLÓGICA I PARTE I CUESTIÓN 16
- PRIZE LIST (ALL PRIZES NON ACQUISITIVE) ALEXANDRA ART EXCELLENCE
- POTASSIUM CHANNELS ANNOTATED BIBLIOGRAPHY AND URLS MISSY CAVALLIN PRIMARY
- REGLER VID TÄVLING FÖR AKTIVA I TFIK GRUPP 1+2+3
- AGENDA PREŞEDINTELUI CONSILIULUI JUDEŢEAN MUREŞ PERIOADA 1 – 31
- DODATAK VII POPIS DOKUMENTACIJE KOJU TREBA PRIKUPITI I DOSTAVITI
- Kiddy Academy Manual Handling Policy as it is
- N REGENTS EARTH SCIENCE EPICENTER LOCATION LAB AME LAB
- SAMODZIELNE STANOWISKO PRACY DO SPRAW INFORMATYKI WYKONUJE NASTĘPUJĄCE ZADANIA
- FEUILLE DE TRAVAIL SUR LES CROYANCES NOM
- INTRODUCTION APPLE COMPUTERS INC WAS FOUNDED IN 1976 BY
- INTRODUCCIÓN A ARCGIS 92 PREPARADA POR DAVID R MAIDMENT
- CATÁLOGO GENERAL TORNOSFRESADORAS CNC MAQUINARIA MADRID SA C VILLAFRANCA
- BR00142242017III PROTOKÓŁ NR 242017 POSIEDZENIA KOMISJI GOSPODARKI RADY MIEJSKIEJ
- L EY DE PROTECCIÓN A PERSONAS DEFENSORAS DE DERECHOS
- POWERPLUSWATERMARKOBJECT357922611 SPECIAL EVENT OBJECTIVES (ICS 202) 1 SPECIAL
- ACTIVIDADES DE CONSTRUCCION MANTENIMIENTO CONSERVACION EXPLOTACION Y OPERACIÓN DE
- SESSION MUSICIAN CONTRACT ARTIST PROJECT NAME SESSION DATE(S)
SURAT PERJANJIAN PELAKSANAAN KEGIATAN MAGANGPKL SISWAMAHASISWA DI LIPI KAMI
SAŽETAK ZA JAVNOST IZMJENA I DOPUNA URBANISTIČKOG PLANA UREĐENJA
THE ROYAL COLLEGE OF PSYCHIATRISTS FACULTY OF LEARNING DISABILITY
MEDICAL SURGE – FLURRICANE 2013 MAY 1316 2013 EXERCISE
ATTACHMENT 16 CERTIFICATION OF SEVERE PHYSICAL DISTRESS I HEREBY
5 NOVANTRONE 10 MG5 ML INJEKCIÓ NOVANTRONE 20 MG10
 INIZIATIVA ARCIMEDICI DEL MONDO SUL CHIAPAS (MESSICO) ALLINTERNO DEL
INIZIATIVA ARCIMEDICI DEL MONDO SUL CHIAPAS (MESSICO) ALLINTERNO DELIMMUNITY FOCUSED CLINICAL ACTIVITY IMMUNITY PNEUMONIA THIS ACTIVITY
[RTFACT21767] DOCKERCOMPOSE UPGRADE FROM ARTIFACTORY 617POSTGRESQL 9611 TO ARTIFACTORY732
 CONTENTS CHAPTER 1 THE COUNCIL OF EUROPE PROJECT ON
CONTENTS CHAPTER 1 THE COUNCIL OF EUROPE PROJECT ONPLANILLA DE PREPARACIÓN MENU DE INVIERNO MENÚ Nº 1
JENISJENIS AYAT AYAT IALAH SUSUNAN KATAKATA YANG MENGANDUNGI MAKSUD
 BEĻĢIJAS POSMA REZULTĀTI 1 HÄNNINEN JUHO MARKKULA
BEĻĢIJAS POSMA REZULTĀTI 1 HÄNNINEN JUHO MARKKULANZQA UNIT STANDARD 23645 VERSION 3 PAGE 2 OF
REPEATBLOCKAMENDAMENDDATE{21102015}21102015DATE ANOA80249ANONUMAM131NUMAM AMENDMENT NUMAM131NUMAM REPEATBLOCKBYMEMBERSJADWIGA WIŚNIEWSKA JANUSZ WOJCIECHOWSKI AND
 REPUBLIKA HRVATSKA ŠIBENSKOKNINSKA ŽUPANIJA OPĆINA TRIBUNJ OPĆINSKO VIJEĆE NA
REPUBLIKA HRVATSKA ŠIBENSKOKNINSKA ŽUPANIJA OPĆINA TRIBUNJ OPĆINSKO VIJEĆE NA DOCUMENT OF THE WORLD BANK FOR OFFICIAL USE ONLY
DOCUMENT OF THE WORLD BANK FOR OFFICIAL USE ONLY EL SMM REVELA QUE UNA GRAN PARTE DE LAS
EL SMM REVELA QUE UNA GRAN PARTE DE LASZAŁĄCZNIK NR 4 DO SIWZ (PIECZĘĆ WYKONAWCY) DOT
INSTRUCCIONS PER A LA CORRECTA EXECUCIÓ DELS SERVEIS EXTERNS