NAME DATE PERIOD DOUBLE REPLACEMENT REACTION
COVERAGE PERIOD [SEE INSTRUCTIONS] S UMMARY OF0 21415 PERIODO OCHENTA Y SEIS DE
0 32737 PERIODO CIENTO CATORCE DE SESIONES
0 43752 PERIODO OCHENTA Y CINCO DE
0 43752 PERIODO OCHENTA Y CINCO DE
19 PERIODO CIENTO TRES DE SESIONES ORDINARIAS
Name _________________________________________________________ Date _______________ Period _________
Double Replacement Reaction
Problem: How does a double-replacement reaction occur?
Background: Some of the most impressive chemical reactions are double-replacement reactions, also called ion-exchange reactions. In a double-replacement reaction, a clear solution of an ionic compound is added to a clear solution of another ionic compound. The positive ions of one compound react with the negative ions of the other compound to form a precipitate, a gas, or water. When a precipitate forms, the result can be very dramatic. The precipitate is an insoluble solid substance that my produce a sudden bright color in the remaining clear solution.
In this investigation you will observe a double-replacement reaction, perform a double-replacement reaction and interpret the results.
Demonstrations/Hypothesis: The teacher will prepare two clear solutions of hydrochloric acid and ammonium hydroxide. Both of these solutions evaporate very rapidly into the air. Hypothesize as to what you think will happen as these two ionic solutions come in close contact with each other in the air. Complete the following equation showing the products expected from this demonstration. Record your observations in Data Table 1.
HCl + NH4OH _________________ + ____________________
Materials:
Safety goggles 1 test tube & rack
2 100 mL beakers Sodium Iodide (Na+1 I -1)
250 mL beaker Distilled Water
3 clean droppers Lead Nitrate (Pb+2 NO3-2)
Procedure:
Place 1 dropper full of distilled water into the test tube.
Carefully measure out ½ dropper full of lead nitrate and drop into the test tube.
Hold the test tube out of the test tube rack.
Carefully measure out ½ dropper full of sodium iodide and drop into the same test tube.
Record your observations in the data table. Be very specific as to what you see.
Let the solution stand in the rack for 10 minutes. Record your observations. Be specific.
Do not spill contents of test tube down the drain! Your teacher will dispose it.
Observations:
|
Solution |
Appearance |
Final Results |
Observations about reaction rate |
|
Hydrochloric Acid |
|
|
|
|
Ammonium Hydroxide |
|
||
|
Lead Nitrate |
|
|
|
|
Sodium Iodide |
|
Analysis/Conclusions:
What ions were present in the lead nitrate solution? (Symbol and charge for each ion) ___________________________________________________________________________________________
What ions were present in the sodium iodide solution? (Symbol and charge for each ion)
___________________________________________________________________________________________
Write a balanced equation for the reaction that took place between the two substances
__________________________________________________________________________________________
How can you account for the change in appearance of the resulting mixture after the 10 minute waiting period? What happened?
___________________________________________________________________________________________
___________________________________________________________________________________________
___________________________________________________________________________________________
Compare and contrast the reactions between hydrochloric acid/ammonium hydroxide and sodium iodide/lead nitrate. Discuss the reactants, products and reaction rates.
______________________________________________________________________________________________
______________________________________________________________________________________________
______________________________________________________________________________________________
______________________________________________________________________________________________
2 PERIODO CIENTO TREINTA Y CUATRO DE
2 PERIODO NOVENTA Y CUATRO DE SESIONES
2 PERIODO NOVENTA Y TRES DE SESIONES
Tags: double replacement, period, reaction, double, replacement
- ZAŁĄCZNIK NR 2 SZABLON PROJEKTU DOKUMENTACJI PLANU DOKUMENTACJA PLANU
- PROCEDURES WHERE A CANDIDATE SEEKS A REVIEW OF A
- EQUAL OPPORTUNITY POLICY THIS MODEL IS FOR GUIDANCE ONLY
- EL CENTAURO LEOPOLDO ALAS LEOPOLDO ALAS EL CENTAURO
- ANESZTEZIOLÓGIA SZÖVŐDMÉNYADATOK TECHNIKAI KÓD MEGNEVEZÉS ÖSSZES 1 DISCONNECTIO 2
- ADOPTED – JANUARY 26 2021 AGENDA ITEM NO 41
- GEOMETRIC ROUTINES OF PTC THE GEOMETRIC ROUTINES OF PTC
- FICHA TECNICA SELLANTES DE FOTOCURADO ASIAMBEXTODOFIT042 FECHA ACTUALIZACIÓN 08062018
- 8 THÔNG BÁO CỦA NGÂN HÀNG NHÀ NƯỚC VIỆT
- ALIMENTAZIONE DOPO IL PRIMO ANNO DI VITA TRA LA
- 80 INTERIOR CASA DE JIMMIE – POR LA MAÑANA
- PROYECTO DE LEY N°760 2000CR PRESENTADO POR EL PODER
- LOS SISTEMAS DE NUMERACION DE LA ANTIGÜEDAD EL SISTEMA
- 8 Z A K O N O POTVRĐIVANJU SPORAZUMA
- PARKER GAMBINO ADDRESS 63 TONETTA LAKE WAY PHONE (HOME)
- TOURNAMENT POCKET BILLIARDS WORLD STANDARDIZED RULES OF THE WORLD
- 84110EA070111 PAGE 0 OF 3 PAGE 1 OF 3
- 8 MARCA 2012 BZ2711222012II O G Ł O S
- EXCMO AYUNTAMIENTO DE CÁDIZ CONCEJALÍA DE LA MUJER FUNDACIÓN
- CHILD & FAMILY DEVELOPMENT PROGRAMS COMMUNITY ACTION TEAM FORM
- REPUBLICA MOLDOVA CONSILIUL RAIONAL HÂNCEŞTI DIRECŢIA ÎNVĂŢĂMÎNT HÂNCEŞTI 3401
- READING IS FUNDAMENTAL AGRICULTURE RELATED TOPICS READING LIST
- YÖNETİM KURULU ADAYLIĞI BEYANI SERMAYE ŞIRKETLERININ GENEL KURUL
- 8 DANH MỤC VĂN BẢN QUY PHẠM PHÁP LUẬT
- COMMENTS WITH REGARD TO NATIONAL EXPERIENCE IN KOREA
- REKTOR ZARZĄDZENIE WEWNĘTRZNE 442020 Z DNIA 5
- 14 PICTOGRAMAS DE PELIGROSIDAD EN LAS ETIQUETAS DE ALGUNOS
- BALANCESKEMA AKTIVER I IMMATERIELLE AKTIVER 1 DRIFTSMIDLER 2 DOMICILEJENDOMME
- INSTRUCTIVO CAMBIO DE DOMICILIO (CASA MATRIZ O SUCURSAL) CAMBIO
- SLUŽBA ZA ZAJEDNIČKE POSLOVE KLASA 40607100107 URBROJ 214001131005 U
LACK OF INFLUENCE OF COMT AND NET GENES VARIANTS
2004IV SETTORESERVSOC194 OGGETTO CONVENZIONE TRA IL COMUNE DI PARMA
 CALWORKS FACT SHEET CONTINUED… CALWORKS PROGRAM FACT SHEET JULY
CALWORKS FACT SHEET CONTINUED… CALWORKS PROGRAM FACT SHEET JULY a-level-french-grammar-booklet-a-level
a-level-french-grammar-booklet-a-levelUNIT PLANNING MATRIX ELEMENTARY AUDIENCE FIFTH GRADE KEY QUESTION
MEGBÍZÁSI SZERZŐDÉS MELY LÉTREJÖTT EGYRÉSZRŐL A MAGYAR LABDARÚGÓ
 SCT95 PÁGINA 8 OMPI S SCT95 ORIGINAL INGLÉS FECHA
SCT95 PÁGINA 8 OMPI S SCT95 ORIGINAL INGLÉS FECHA THIS DOCUMENT WAS CLASSIFIED AS OFFICIAL SAFEGUARDING ADULTS ALERTER
THIS DOCUMENT WAS CLASSIFIED AS OFFICIAL SAFEGUARDING ADULTS ALERTER2 PATVIRTINTA NACIONALINĖS TEISMŲ ADMINISTRACIJOS DIREKTORIAUS 2017 M BIRŽELIO
 POLICY 112 EYEWITNESS IDENTIFICATION EYEWITNESS IDENTIFICATION POLICY & PROCEDURE
POLICY 112 EYEWITNESS IDENTIFICATION EYEWITNESS IDENTIFICATION POLICY & PROCEDURE TÜRKIYEDEKI DONÖR UYGULAMALARI VE STKLARA KAYNAK SAĞLAMA STGP EYLÜL
TÜRKIYEDEKI DONÖR UYGULAMALARI VE STKLARA KAYNAK SAĞLAMA STGP EYLÜLBILGISAYAR MÜHENDISLIĞI EĞITIMINDE MATEMATIK ALTYAPI VE TARIHE BAŞVURUNUN ÖNEMI
 ACTA N° 2 REUNIÓN COMITÉ DE COORDINACIÓN
ACTA N° 2 REUNIÓN COMITÉ DE COORDINACIÓNMODIFICACIONES GESTIÓN DE CENTROS AL 03062009 BACHILLERATO IMPRESIÓN
 BUSINESS PROCESS PROCEDURE TITLE WAGE TYPE REPORTER PROCESS PAYROLL
BUSINESS PROCESS PROCEDURE TITLE WAGE TYPE REPORTER PROCESS PAYROLL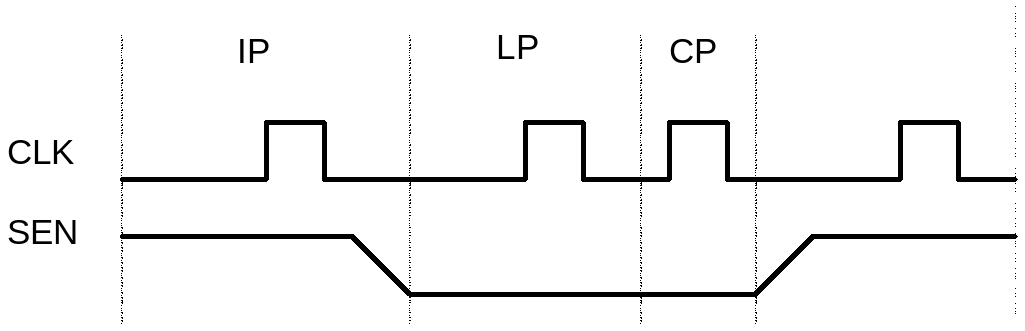 ENHANCING DELAY FAULT COVERAGE THROUGH LOW POWER SEGMENTED SCAN
ENHANCING DELAY FAULT COVERAGE THROUGH LOW POWER SEGMENTED SCANMANUSCRIPT TITLE SPONTANEOUS SEPTIC ARTHRITIS OF CANINE ELBOWS 21
 REAL FEDERACIÓN ESPAÑOLA DE JUDO Y DEPORTES ASOCIADOS JIUJITSU
REAL FEDERACIÓN ESPAÑOLA DE JUDO Y DEPORTES ASOCIADOS JIUJITSUEVALUACIÓN DEL CONFORT TÉRMICO EN MEXICALI BC ANTE EL
 REGLAMENTO DE RÉGIMEN INTERIOR COWORKING BENQUERENCIA EL AYUNTAMIENTO DE
REGLAMENTO DE RÉGIMEN INTERIOR COWORKING BENQUERENCIA EL AYUNTAMIENTO DE