EUROPEAN DIRECTORATE FOR THE QUALITY OF MEDICINES & HEALTHCARE
0 EN EUROPEAN ECONOMIC AND SOCIAL COMMITTEE29 THE SCOPE FOR ACTION OF EUROPEAN PARLIAMENT
3 EUROPEAN ECONOMIC AND SOCIAL COMMITTEE SPEAKING
Е ВРОПЕЙСКИЙ ПРОЕКТ НПМ COUNCIL OF EUROPE EUROPEAN
EUROPEAN COURT OF HUMAN RIGHTS JUDGMENTS ON THE
EUROPEAN CURRICULUM VITAE FORMAT PERSONAL INFORMATION IEVA GRUNDSTEINE
Form to be filled in for each application for a Certificate of Suitability to the monographs of the European Pharmacopoeia in a
European Directorate for the Quality of Medicines & HealthCare
Certification of Substances Department
CHANGE OF CONTACT DETAILS
FOR A CERTIFICATE OF SUITABILITY
Date of notification: ……./……/……
1. General Information
Dossier number and substance
CEP …………………………/ [Substance name] …………………………
Subtitle (if applicable) .…………………………
In case the change concerns several CEPs, please list the dossier numbers and substances here:
CEP …………………………/ [Substance name] …………………………
2. Details of contact person authorised for communication on behalf of the holder:
(if contact is part of a company/group different from holder please provide an authorisation letter - see Annex 1):
|
Title* (Mrs, Mr, Dr) |
|
|
First name* |
|
|
Family name* |
|
|
Job title/Department |
|
|
Name of the company* |
|
|
Address for correspondence* |
|
|
Postcode* |
|
|
Town* |
|
|
Country* |
|
|
Telephone* |
|
|
E-mail* |
|
|
Fields marked * are mandatory
|
|
Annex 1
Template letter of Authorisation
address of the holder
date and place
LETTER OF AUTHORISATION
We, name of the holder, hereby authorise, name of the authorised representative, to act as official representative for our Certificate of Suitability for name of the substance.
Signature
Page
(OPIS W JĘZYKU ZAJĘĆ) MODULE NAME EUROPEAN UNION INTELLECTUAL
0150781 TOYOTA MOTOR EUROPEAN (TME) SUSTAINABLE LOGISTICS AN EXAMPLE
1 GRUPO «EUROPEAN 112 DAY 2016» ACTIVIDAD 112 EXTREMADURAUNIVERSIDAD
Tags: directorate for, healthcare, quality, medicines, directorate, european
- CIRCULAR DE 21 DE MAYO DE 2009 DE LA
- RENAL TERMINOLOGY AND NOMENCLATURE 27710 KELLUM J A ET
- NORTHAMPTONSHIRE CHILDREN’S SERVICES (NCS) PROCEDURES MANUAL PRACTITIONER GUIDANCE NOTES
- 5 RELATIVISM MANY DIFFERENT IDEAS HAVE BEEN GIVEN THE
- VILNIAUS MIESTO SAVIVALDYBĖS ADMINISTRACIJOS KULTŪROS IR UGDYMO DEPARTAMENTO KULTŪROS
- STUDIJ DVOPREDMETNI PREDDIPLOMSKI SVEUČILIŠNI STUDIJ LATINSKOG JEZIKA I RIMSKE
- EL DUEÑO DE LA LUNA ENLACE AL VÍDEO HTTPSWWWYOUTUBECOMWATCH?V6JDYJX9BKKC
- DOCUMENT WSISPC3CONTR56E 31 MAY 2003 ORIGINAL ENGLISH ASSOCIATION FOR
- RESTAURANTE CONCHI RASCAFRÍA SUGERENCIAS TIMBAL DE VERDURAS ASADAS
- ČESKÁ NÁRODNÍ SKUPINA MEZINÁRODNÍ SPOLEČNOSTI PRO TRESTNÍ PRÁVO Z
- LO YOUNGSGATEN 11 0181 OSLO VÅR SAK NR 161508
- 0 RESOLUTION RE STANDARD SECURITY EXTRACT FROM THE MINUTES
- SALARY SACRIFICE WELCOME TO ANOTHER AAT PODCAST WHICH TODAY
- CENTRO CULTURALE S NICOLÒ LECCO ASSESSORATO CULTURA
- FROM SEQUENCE TO TREESAAP DOWNLOADING SEQUENCES FROM NCBI IS
- ELISE MCMAHON EMCMAHONCANINEHEADSTARTCOM 125 EAST CHESTNUT HILL ROAD
- Total Building Minutes Special Notes Weekly Sped Services Schedule
- SISTEMA DE GESTIÓN DE CALIDAD CENTRO UNIVERSITARIO DEL SUR
- EXPERIENCIA DEL PROPONENTE IMPLA UTILIZANDO EL FORMATO PROPUESTO PROPORCIONA
- ЕКОНОМСКОТРГОВИНСКА ШКОЛА ЗАЈЕЧАРКНЕГИЊЕ ЉУБИЦЕ 35 БРОЈ 6111186011 18052020ГОДИНЕ НА
- TEMA I EL DERECHO INTERNACIONAL PRIVADO IPRESUPUESTOS Y OBJETO
- ZLECENIE WYKONANIA BADAŃ ZAKŁAD HIGIENY WETERYNARYJNEJ UL BRYNOWSKA 25A
- APPENDIX B QUESSI CONSTITUTION BYLAW NO 1 A BYLAW
- 10 COSAS QUE DEBE CONOCER AL ADQUIRIR UNA PÓLIZA
- KRAJSKÝ ÚŘAD ZLÍNSKÉHO KRAJE ODBOR DOPRAVY A SILNIČNÍHO HOSPODÁŘSTVÍ
- ANNA M CIENCIALA HISTORY 557 SPRING 2002 WES 4002
- OUTIL 25 FORMULATIONS USUELLES À UTILISER AU TÉLÉPHONE
- LISTEN A MINUTECOM ANIMALS HTTPWWWLISTENAMINUTECOMAANIMALSHTML ONE MINUTE A DAY
- N A C R T TURISTIČKA ZAJEDNICA GRADA ŠIBENIKA
- OREGON SMOKE MANAGEMENT REPORTING SYSTEM CODING SHEET RIGHTOFWAY UNITS
HALAMAN COVER INI SILAKAN DIISI DAN DITEMPELKAN DI BAGIAN
MODEL PRAKTIJKOVEREENKOMST VOOR STAGIAIRS DE ONDERGETEKENDEN 1 DE BEROEPSPRAKTIJKPLAATS
 INSTRUCCIONES PARA CUMPLIMENTAR EL IMPRESO DE SOLICITUD L2
INSTRUCCIONES PARA CUMPLIMENTAR EL IMPRESO DE SOLICITUD L2  MARIESTADS PISTOLKLUBB ANSÖKAN OM INTYG FÖR ”AKTIVT MEDLEMSKAP” I
MARIESTADS PISTOLKLUBB ANSÖKAN OM INTYG FÖR ”AKTIVT MEDLEMSKAP” ISOLO IMPROVISATION PAGE | 8 PERFORMANCE ASSESSMENT DEVELOPMENT TEMPLATE
NOTE THIS AGREEMENT IS A SAMPLE ONLY AND IS
MYPLATE MYGARDEN OVERVIEW A BALANCED DIET IS ESSENTIAL TO
E – CETVELİ BAZI ÖDENEKLERİN KULLANIMINA VE HARCAMALARA İLİŞKİN
TÜRKİYE BANKALAR BİRLİĞİ SUÇ GELİRLERİNİN AKLANMASI VE TERÖRİZMİN FİNANSMANI
TEMPLATE FOR POSTPROJECT REPORT ON SPECIAL EDUCATION ARTISTINSCHOOL (SPEDAISS)
 NAME BLACKPOOL FIELD VISIT ACTIVITIES USEFUL WEBSITE WWWBLACKPOOLGOVUK 1
NAME BLACKPOOL FIELD VISIT ACTIVITIES USEFUL WEBSITE WWWBLACKPOOLGOVUK 1 TÁJÉKOZTATÓ KOLLÉGIUMI FÉRŐHELY IGÉNYLÉSÉHEZ SZÜKSÉGES IGAZOLÁSOKRÓL A PÁLYÁZATI ADATLAP
TÁJÉKOZTATÓ KOLLÉGIUMI FÉRŐHELY IGÉNYLÉSÉHEZ SZÜKSÉGES IGAZOLÁSOKRÓL A PÁLYÁZATI ADATLAP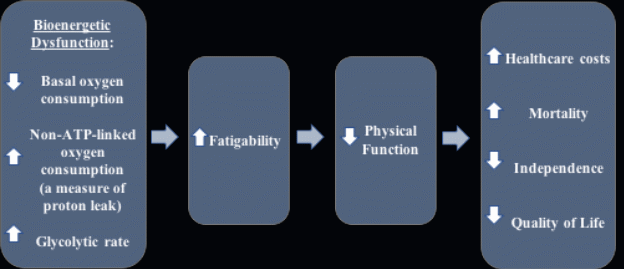 THE EFFECTS OF AGING ON PLATELET BIOENERGETICS BY CATHERINE
THE EFFECTS OF AGING ON PLATELET BIOENERGETICS BY CATHERINEREKLAMCILIK (NÖ) BÖLÜMÜ DERS MÜFREDATI 1 DÖNEM KREDİSİ AKTS
OBSAH 1 TEORIE A PROJEKTOVÁNÍ V TEPELNÉ TECHNICE 11
 ANNEX II ERASMUS INTENSIVE LANGUAGE COURSES 201314 ORGANISING
ANNEX II ERASMUS INTENSIVE LANGUAGE COURSES 201314 ORGANISINGPRESSEMEDDELELSE KØBENHAVN DEN 14 JUNI 2010 G4S LANCERER MARKEDETS
 TORNEO GRAVN FEDERACIONES 2010 GRAVN FEDERAZIOEN ARTEKO TXAPELKETA 2010
TORNEO GRAVN FEDERACIONES 2010 GRAVN FEDERAZIOEN ARTEKO TXAPELKETA 2010 ACİL BATIN RADYOLOJİSİ YARD DOÇ BENGI GÜRSES YEDITEPE ÜNIV
ACİL BATIN RADYOLOJİSİ YARD DOÇ BENGI GÜRSES YEDITEPE ÜNIVNR SPRAWY 4II2 ZAŁĄCZNIK NR 2 DO SIWZ NR