PROCEDURE 51 LOW RISK RESEARCH RESEARCH GOVERNANCE UNIT ST
ALL SCHOOL PROCEDURES AND POLICIES FROM THE STUDENTDISCHARGE OF CARE ORDERS – STANDARD PROCEDURE THE
ILLICIT DISCHARGE DETECTION AND ELIMINATION FIELD PROCEDURES AND
OFFICE OF STUDENT EMPLOYMENT PROCEDURE FOR GRADUATE
Procedures for Varying Shared Ownership Leases Background
YOUTH OFFENDING SERVICE HOME VISITLONE WORKING PROCEDURE
Patient Admission
Procedure 5.1
Low Risk Research
|
|
RESEARCH GOVERNANCE UNIT St. Vincent’s Hospital (Melbourne) Caritas Christi Hospice St. George’s Health Service Prague House |
|
|
|
LOW RISK RESEARCH
Statement of Intent and Outcomes
The St Vincent’s Hospital Human Research Ethics Committee is committed to fulfilling Section 5 of The National Statement on Ethical Conduct in Human Research (2007) to ensure that the review of low and negligible risk research activities are expedited, and that such research receives appropriate research governance at all times.
Definitions
Low Risk Research is defined as research in which the only foreseeable risk is one of discomfort.
Negligible Risk Research is defined as research in which there is no foreseeable risk of harm or discomfort, and any foreseeable risk is of inconvenience only.
Research Governance is defined as the regulations, principles and standards of good practice that exist to achieve, and continuously improve, research quality.
Procedure
All members of the St Vincent’s Hospital Human Research Ethics Committee must be familiar with The National Statement on Ethical Conduct in Human Research (2007), and in particular, Section 5.19.
To maximise efficiency and reduce resource expenditure, an expedited approval pathway is offered to low and negligible risk research.
This pathway involves review by a Low Risk Research Subcommittee. The Subcommittee is comprised of members from the HREC who are selected by their level of expertise to participate in an online review process. A minimum of four reviewers will be selected (including at least one lay member), and asked to review the Low Risk Research Application. All Subcommittee members must be afforded the opportunity to formally comment on the Low Risk Research Application and any comments by other reviewers prior to its approval.
Once comments are received, the Research Governance Unit will provide the Principal Investigator with formal written correspondence approving the application, requesting additional information/clarification or rejecting the application. The Subcommittee retains the right to refer any project to review by the full HREC meeting, but must justify this to the Principal Investigator in a timely manner.
All comments from the Low Risk Research Subcommittee will be documented and filed within the Research Governance Unit. All decisions of the Subcommittee must be formally noted at the next available HREC meeting and formally documented within the minutes.
A governance review will also be undertaken by the Research Governance Unit.
To be considered via the Low Risk Research pathway, the research must not include:
Women who are pregnant
Children or young people under the age of 18
Persons with an intellectual disability or mental impairment of any kind
Persons incompetent to provide informed consent
People involved in illegal activities
Prisoners or people on parole
Research specifically recruiting Aboriginal and / or Torres Strait Islander people
Persons not usually considered to be vulnerable but would be considered vulnerable in the context of this research project
Establishment of a databank for research purposes
Interventions and therapies including clinical and non-clinical trials and innovations
Human genetic research or gene technology
Derivation or use of human stem cells
Deception of participants, concealment or covert observation
Radioactive substances / ionizing radiation e.g. X-rays, DEXA
Assisted reproductive technology (ART)
Xenotransplantation
Toxins / mutagens / teratogens / carcinogens
Associated Procedures/Instructions
Procedure 2.1 – Assessment of Risks and Benefits
Procedure 2.2 – Obtaining and Honouring Consent
Procedure 2.3 – Qualifying or waiving conditions for consent
Reference Documents
The National Statement on Ethical Conduct in Research Involving Humans in accordance with the NHMRC Act, 2007 (Cth).
Australian Code for the Responsible Conduct of Research (2007)
Authorized by:
Dr Megan Robertson
Director of Research
|
Author: Dr Tam Nguyen, Executive Officer |
|
|
Date Issued: 2011 |
Next Review: 2017 |
|
Date Revised: 2015
|
Filepath: |
5.1 Low
Risk Research Page
(REPORT TEMPLATE) INDEPENDENT ACCOUNTANT’S REPORT ON APPLYING AGREEDUPON PROCEDURES
(YOUR BUSINESS NAME HERE) – SAFE WORK PROCEDURE
(YOUR BUSINESS NAME HERE) – SAFE WORK PROCEDURE PORTABLE
Tags: research research, risk research, research, governance, procedure
- EL MONUMENTO A CRISTOBAL COLÓN DE BARCELONA EN CONSTRUCCIÓN
- BAS03 APPLICATION FOR THE REGISTRATION OF THIRD PARTY
- 10 FTKOKONICA MINOLTAKONICA MINOLTA C353 VS KYOCERA KMC4035E PAGE
- PARANÁ 14 DE FEBRERO DE 2012 AL SENADOR PROVINCIAL
- ALNUS GLUTINOSA ALISO FAMILIA BETULACEAE NOMBRE COMÚN ALISO LUGAR
- USNESENÍ XII SJEZDU DELEGÁTŮ ČLK KONANÉHO VE DNECH 8
- REPUBLIKA HRVATSKA OSJEČKOBARANJSKA ŽUPANIJA OPĆINA DARDA NAČELNIK KLASA 302012101
- SUAP SPORTELLO UNICO ATTIVITÀ PRODUTTIVE VIA DI FRANCIA 1
- VO2 SAMPLE PROBLEMS ADVANCED PHYSIOLOGY OF EXERCISE 1 1
- DIRECTIONS 1 TYPE THE WORD LIST BELOW USING A
- SUPPLEMENTARY FIGURE AND TABLE LEGENDS SUPPLEMENTARY FIGURE S1 GENERATION
- Whole School Policy for Safeguarding Incorporating Child Protection Thomas
- PRELIMINARY ACTIVITIES PRELIMINARY ACTIVITIES 1º CONSTITUTION
- 488 SAYILI DAMGA VERGİSİ KANUNU (1964) MÜKELLEFIYET VE İSTISNALAR
- EXPOPAZ EL EVENTO DEL AÑO EN COLOMBIA EN CONSTRUCCIÓN
- M 1 ANATOMY RICHARD E BURNEY MD DECEMBER 20
- EDITAL CONCURSO FOTOGRÁFICO UMA UNIVERSIDADE VÁRIOS OLHARES – A
- PATVIRTINTA VALSTYBĖS GARANTUOJAMOS TEISINĖS PAGALBOS TARNYBOS DIREKTORIAUS 2015 M
- INTRODUCCIÓN A LA BIOLOGÍA CELULAR Y MOLECULAR IBCM TURNO
- CIRCULAR Nª 47 DEL 14 DE AGOSTO DE 1998
- TRANENT MEDICAL PRACTICE PRACTICE PROFILE AS AT JANUARY
- INTRODUCCIÓN EL AAL ES UN BIFOSFONATO CUYO MECANISMO DE
- BERITA ACARA MONITORING DAN EVALUASI PROSES PEMBELAJARAN SEMESTER GASAL
- BIOGEOCHEMICAL CYCLES WATER NITROGEN AND CARBON A CONDENSATION
- 0 DOCUMENTO DE REFLEXIÓN DE LA COMISIÓN
- BARDU KOMMUNE PLEIE OG OMSORGSTJENESTEN PARKVEIEN 24 9360
- UNIVERSIDADE DO VALE DO RIO DOS SINOS CONSELHO UNIVERSITÁRIOCPGPEX
- SOLICITUD DE ADQUISICIÓN DE NAVES PARQUE EMPRESARIAL NOMBRE
- ANNEX II BIOGRAPHICAL DATA FORM OF CANDIDATES TO HUMAN
- LUGAR DE REALIZACIÓN DE LOS EXÁMENES CONVOCATORIA EXTRAORDINARIA DICIEMBRE
 RELAY LINE DRILL PURPOSE TO IMPROVE RELAY TECHNIQUE EQUIPMENT
RELAY LINE DRILL PURPOSE TO IMPROVE RELAY TECHNIQUE EQUIPMENT CORRECCIÓN AL ANEXO 1 BIOX ANEXO 1 BIS MODELO
CORRECCIÓN AL ANEXO 1 BIOX ANEXO 1 BIS MODELO P RESUPUESTO PARA EJECUCIÓN DE OBRAS MENORES PRESUPUESTO QUE
P RESUPUESTO PARA EJECUCIÓN DE OBRAS MENORES PRESUPUESTO QUE4 MELLÉKLET …2009 (… …) IRM RENDELETHEZ KÖZBESZERZÉSI ÉRTESÍTŐ
SCHEDA DI ANALISI GUIDATA DEL TESTO NARRATIVO TITOLO
 OBRADA I ANALIZA PODATAKA 1 – RADNA SKRIPTA BOŠKO
OBRADA I ANALIZA PODATAKA 1 – RADNA SKRIPTA BOŠKOOSMOSIS & DIFFUSION LAB – DIALYSIS TUBING BACKGROUND
ELI LILLY ANNOUNCES HUMULIN® N AND 7030 PENS PHASE
II2 CANTIDAD DE CARGOS A COBRAR SEGÚN RUTA O
VAMOS BONITA DIÁLOGOS CARMEN TRAE OTRA
 TRIBUNAL SUPERIOR DEL DISTRITO JUDICIAL DE BUGA COMUNICADO TRIBUNAL
TRIBUNAL SUPERIOR DEL DISTRITO JUDICIAL DE BUGA COMUNICADO TRIBUNAL ZAŁĄCZNIK NR 4 DO SWZ NUMER SPRAWY KRKWAD27142021 ZAMAWIAJĄCY
ZAŁĄCZNIK NR 4 DO SWZ NUMER SPRAWY KRKWAD27142021 ZAMAWIAJĄCY4 KOMUNIKACJA SPOŁECZNA I PODEJMOWANIE DECYZJI KOMUNIKACJA SPOŁECZNA
LEY 448 DE 1998 (JULIO 21) DIARIO OFICIAL NO
ZAŁĄCZNIK IB – ZAŚWIADCZENIE ORGANU ODPOWIEDZIALNEGO ZA MONITOROWANIE OBSZARÓW
APRUEBA LA LEY DE REESTRUCTURACIÓN EMPRESARIAL DECRETO LEY Nº
DEL A HANDY 1 BEGREPER HANDY KORTLESER
PLEASE SPECIFY FILE NAME FOR DOWNLOAD
 VERZOEK AAN HET WEDSTRIJD PROTESTCOMITÉ WIJZIGING ZEILNUMMER ANDER
VERZOEK AAN HET WEDSTRIJD PROTESTCOMITÉ WIJZIGING ZEILNUMMER ANDER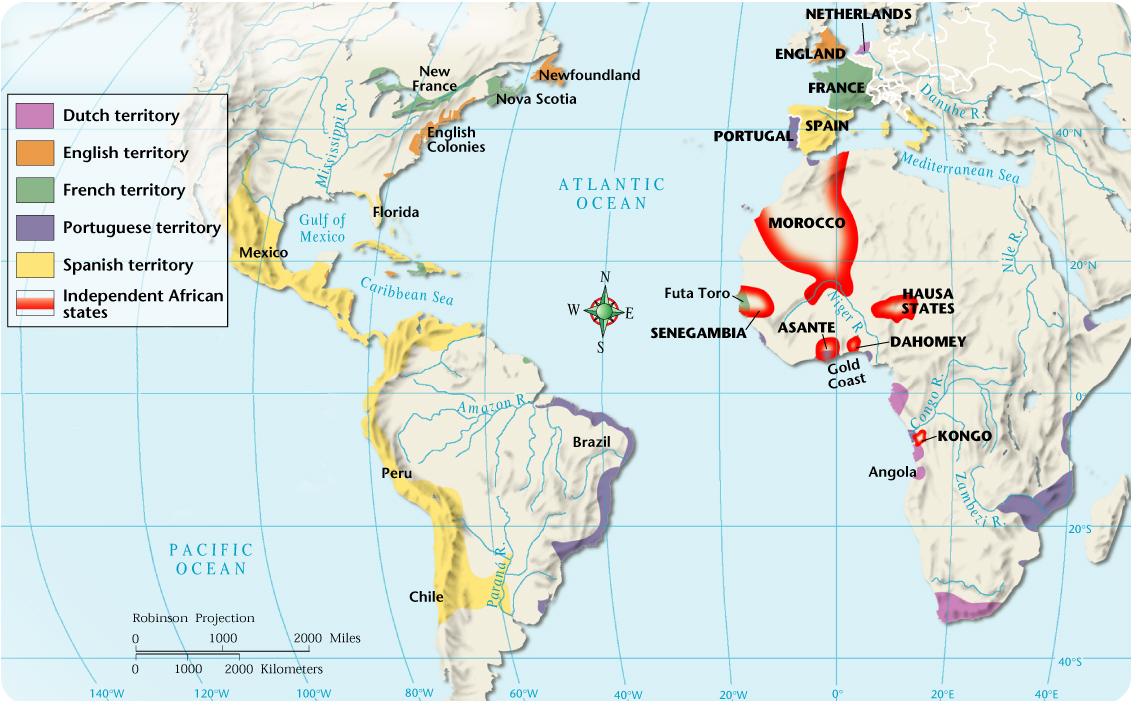 CONQUEST IN THE AMERICAS VARIOUS FACTORS ENABLED THE
CONQUEST IN THE AMERICAS VARIOUS FACTORS ENABLED THE
