L ABORATORY INVESTIGATION TITRATION OF AN UNKNOWN ACID
BASES LABORATORY ACCREDITATION GUIDELINES RATIONALE AN ACCREDITEDGENERAL RISK FORM ASSESSMENT GUIDANCE – LABORATORY INTRODUCTION
0 HELSINKI UNIVERSITY OF TECHNOLOGY SYSTEMS ANALYSIS LABORATORY RESEARCH
1 BIOLOGY LABORATORY LABORATORY SAFETY OBJECTIVE LEARN SAFETY
1 INTRODUCTION 11 SCOPE OF THE LABORATORY THE DUBLIN
1 SOFTWARE ENGINEERING LABORATORY SIGNUP SHEET CS 2002
Finding the Size of a Molecule:
L
 aboratory
Investigation:
aboratory
Investigation:
Titration of an Unknown Acid
Purpose:
To prepare a solution of NaOH, and to standardize that solution.
To use titration to determine the concentration of a solution of hydrochloric acid of unknown concentration.
Introduction:
In acid-base titration, an acid and base are reacted in order to determine the concentration of one or the other. In this lab we will react a solution of NaOH that we prepare with solid oxalic acid, H2C2O4, and with acid of an unknown concentration. The titration with the solid oxalic acid will be used to determine our NaOH solution concentration. Once we have standardized the NaOH solution, we will then determine the unknown acid concentration.
Materials:
Solid NaOH Oxalic Acid Dihydrate(H2C2O4. 2H2O) Buret
Volumetric Flask Erlenmeyer flask Wash Bottle Indicator Solution Hydrochloric Acid of unknown concentration
Procedure:
A. Preparation of a Solution of NaOH
1. Obtain a 100 mL volumetric flask and fill it half way with deionized water.
2. Calculate the mass of NaOH needed to make 100.0 mL of a 0.250 M solution of NaOH. Check your answer with your instructor before proceeding.
3. Mass out as close to this value as you can using a piece of weighing paper for the solid. Record the exact mass of NaOH used on your data sheet. You will use your this value as your “true value” to determine the percent error of your NaOH solution.
4. Immediately add your solid NaOH to the water in the volumetric flask. Put the top on the flask and mix as directed until the base has dissolved.
5. Use your wash bottle and add deionized water until the meniscus of the solution reaches the line on the flask.
6. Put the top on the flask and mix the solution again.
B. Standardization of the NaOH
1. Mass out as close to 0.20 g of oxalic acid dihydrate (H2C2O4·2H2O) as you can using a piece of weighing paper. Record the exact value used on your data sheet. Do not exceed .25 g of solid acid!
2. Add the oxalic acid dihydrate to an Erlenmeyer flask. Add approximately 25 mL of deionized water to the flask, and dissolve the acid by swirling gently. If not all of it dissolves, it will dissolve as the titration proceeds.
3. Rinse the buret on the “B” side with deionized water. Add the NaOH solution (from Part A) to the buret. Fill the buret to as close to the 0.00 mark as you can. Open the stopcock and let the tip fill with NaOH solution. Read the buret to the closest 0.01 (remember to read the buret directly from the top down) and record the reading on your data sheet.
4. Add 3 DROPS (too much indicator will cause cloudy solutions!) of the indicator to the flask, and titrate the solid acid with your NaOH. Try to obtain the lightest permanent shade of pink in the flask that you can. Use your wash bottle to rinse the sides of the Erlenmeyer. Remember to record starting and final volumes for the base on your data sheet.
5. Repeat the titration using a second sample of oxalic acid dihydrate. Again, record the mass of the solid, and starting and initial volumes of the base on your data sheet. If these two trials are off by more than 10%, repeat the titration, and then average the two closest trials.
C. Determination of the Molarity of an Unknown
1. Obtain a solution of HCl with an unknown molarity from your instructor. Note the letter for the unknown on your data sheet.
2. Rinse the buret on the “A” side with deionized water. Add the HCl solution to this buret until it is about half full. Again, open the stopcock to fill the tip with acid solution. Read the buret to the closest 0.01. Read the level on your base buret.
3. Put between 10 and 11 mL of HCl into the Erlenmeyer.
4. Add 3 drops of the indicator to the flask, and titrate HCl with your NaOH. Try to obtain the lightest permanent shade of pink in the flask that you can.
5. If your solution turns dark pink, add acid dropwise until it turns clear again. Then, titrate with base until you achieve a light pink color.
6. Remember to record starting and final volumes for the acid and base on your data sheet. Repeat the titration until you obtain at least two good endpoints.
Calculations:
Part B.
1. From the mass of the solid oxalic acid dihydrate, calculate the number of moles of acid used in each titration. Hint: since this is a dihydrate, remember to include the mass of water!
2. Using the balanced equation for the neutralization (below), calculate the number of moles of base used in each titration.
H2C2O4 + NaOH ?
3. Knowing the volume of the NaOH used, calculate the molarity of the NaOH for each trial.
4. Average the two trials to find the average experimental value for your NaOH.
5. Calculate the value for the molarity of the NaOH using the mass of NaOH you measured. Calculate percent error using this as your true value.
Part C.
1. For each trial, calculate the volumes of acid and base used.
2. Use these volumes and the molarity of your base obtained in the calculations above to calculate the molarity of the unknown acid.
DATA SHEET
Part A.
|
Mass of NaOH used in solution |
|
Part B.
|
|
Trial #1 |
Trial #2 |
Trial #3 |
|
Mass of Oxalic Acid |
|
|
|
|
Initial Buret reading |
|
|
|
|
Final Buret reading |
|
|
|
|
Volume of NaOH required |
|
|
|
Part C.
Unknown Acid________________________________________________
-
Trial #1
Trial #2
Initial Buret reading—acid
Final Buret reading—acid
Initial Buret reading—base
Final Buret reading--base
Lab Writeup in Lab Notebook:
1. Data Tables, including units.
2. Calculations—neatly written out, including proper sig figs and units.
3. The answer for the molarity of your unknown acid—circle this number on bottom of page of calculations. There will be an accuracy grade for this part of the lab!
4. Error Analysis
5. Conclusion
10122021 26 SEDIMENT LABORATORY PROCEDURES REDWOOD SCIENCES LABORATORY USDA
117 ACCREDITATION NO ISO 15189 TESTING LABORATORYMEDICAL DEPARTMENT 11704
12 ĆWICZENIE LABORATORYJNE NR 4 (W24) 4 022010
Tags: aboratory investigation:, unknown, investigation, titration, aboratory
- DRAFT CODE OF OFFENCES AGAINST THE PEACE AND SECURITY
- THE PARNITHA (ATTIKI) EARTHQUAKE OF SEPTEMBER 7 1999 APPLICATION
- TOWNSHIP OF ATHENS PUBLIC LIBRARY T OWNSHIP OF ATHENS
- CURRICULUM CHEMISTRYACS APPROVED (MIN 180 TOTAL CREDITS 60 UPPER
- 8 PRUEBA DE CONTROL MODELO B NOMBRE FECHA GROUP
- AÑO 2001 ELECTRONICA INDUSTRIAL TRABAJOS PRACTICOS EJERCICIO
- BASICS ON MACANESE MATRIMONIAL FINANCES PRESENTED BY PAULA NUNES
- CURRICULUM VITAE PERSONAL DATA NAME MARC PETERSGOLDEN MD EDUCATION
- OBAVEZA BROJ 1 UNAPREĐENJE PRUŽANJA KOMUNALNIH USLUGA I SERVISA
- ПРИЛОЖЕНИЕ № 1 К РЕШЕНИЮ КОЛЛЕГИИ ЕВРАЗИЙСКОЙ ЭКОНОМИЧЕСКОЙ КОМИССИИ
- DECRETO POR EL QUE SE CREA CON CARÁCTER DE
- 4 PATVIRTINTA LIETUVOS RESPUBLIKOS VYRIAUSIOSIOS RINKIMŲ KOMISIJOS 2016 M
- CONTOH UNTUK CPNS TRENGGALEK JULI 2021 KEPADA YTH BAPAK
- PART 7 ELECTRIC CONDUCTIVITY OF SOLUTIONS 15 PART 7
- pquimicos_compras
- FORMULARIO DE SOLICITUD PARA LA APROBACIÓN DE EXPORTACIÓN DE
- SERVO PRODUCT DATA SHEET SERVO RR G RADES
- TABLE OF CONTENTS CHAPTER 1 PURPOSE CONTENT AND CONTEXT
- KPAHMOFM004 KENYA PORTS AUTHORITY SHIPPING MOVEMENT MOMBASA PORT POSITION
- 6 EXPTE Nº 655218 VISTO EL CONVENIO A CELEBRAR
- WYMAGANIA EDUKACYJNE NIEZBĘDNE DO UZYSKANIA ŚRÓDROCZNYCH I ROCZNYCH OCEN
- N OTA DE PRENSA VER A LOS AMIGOS CELEBRAR
- ATLANTIC WHITE CEDAR (CHAEMAECYPARIS THYOIDES) SOME AUTHORS WRITE IT
- ‑ 0 ‑ RÉUNION DES MINISTRES DE LA JUSTICE
- QUÍMICA DEL CARBONO PROBLEMAS RESUELTOS (OVIEDO 20192020JUNIO5A B) IDENTIFIQUE
- El Camino de Santiago Preguntas Preguntas… Forma Frases Correctas
- ANALIZA SYTUACJI ROLNICTWA I OBSZARÓW WIEJSKICH WOJEWÓDZTWA ZACHODNIOPOMORSKIEGO POD
- 338 MARY – OUR MOTHER AND MODEL OF OUR
- KERANGKA ACUAN KERJA (KAK) KEGIATAN PENINGKATAN MANAJEMEN ASET PEMERINTAH
- F CON FECHA 24 DE ABRIL DEL PRESENTE AÑO
ANEXO IV CUADRO ALUMINARIAS POSICIÓN DESCRIPCIÓN PRECIO 1 LUMINARIA
SOLICITUD LICENCIA DE PRIMERA OCUPACIÓN DDª MAYOR DE
2 FORMA PATVIRTINTA DRUSKININKŲ SAVIVALDYBĖS ADMINISTRACIJOS DIREKTORIAUS 2018 M
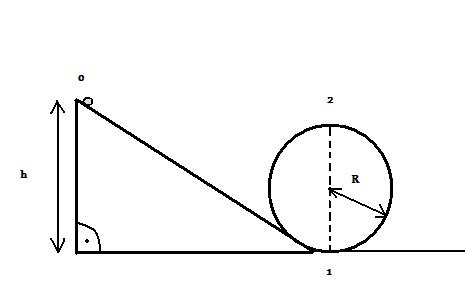 ZÁKON ZACHOVANIA ENERGIE – GULIČKA NA KRUHOVEJ DRÁHE MICHAL
ZÁKON ZACHOVANIA ENERGIE – GULIČKA NA KRUHOVEJ DRÁHE MICHALMISSION CREEK GOLF COURSE 2021 TOURNAMENT CONTRACT CONTACT INFORMATION
LITANIES IN HONOR OF THE BLESSED SACRAMENT KYRIE ELEISON
 TISZTELT SPORTOLÓK EDZŐK SPORTORVOSOK SPORTVEZETŐK TESTNEVELŐK ÉS SPORTBARÁTOK! KÖZÖS
TISZTELT SPORTOLÓK EDZŐK SPORTORVOSOK SPORTVEZETŐK TESTNEVELŐK ÉS SPORTBARÁTOK! KÖZÖSCOMO HABLAR DE DIFERENCIAS EN UN MUNDO INDIFERENTE ?
 OFFICIAL USE ONLY INDEX NO PX53B (WHEN INFORMATION FILLED
OFFICIAL USE ONLY INDEX NO PX53B (WHEN INFORMATION FILLED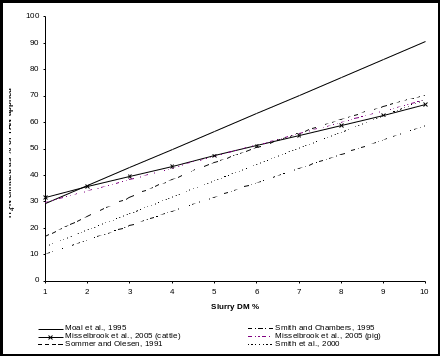 AMMONIA EMISSIONS FROM LAND APPLICATION OF DILUTE SLURRY EXPERT
AMMONIA EMISSIONS FROM LAND APPLICATION OF DILUTE SLURRY EXPERTÚŘAD MĚSTSKÉ ČÁSTI PRAHA 14 ODBOR OBČANSKOSPRÁVNÍ ŽÁDOST O
CHINA SHANSHUI CEMENT GROUP LTD 00691 THE EXCHANGE HAS
KENMERK XXXXXXPA&O JULI 2008 CONVENANT CVB – UR
TEMA 23 LA RESPONSABILIDAD PATRIMONIAL DE LA ADMINISTRACION LOS
 MANUALANVISNING – INLOGGNING I PILUDDENS BÅTKLUBBS ADMINISTRATIVA SYSTEM –
MANUALANVISNING – INLOGGNING I PILUDDENS BÅTKLUBBS ADMINISTRATIVA SYSTEM –OMB 25060159 (EXP 5312008) APPLICATION KIT LOAN
 LABORATUAR NUMUNE RED KAYIT FORMU DOKÜMAN NO BLMLFR01TL05 YAYIN
LABORATUAR NUMUNE RED KAYIT FORMU DOKÜMAN NO BLMLFR01TL05 YAYIN TÍTULO ¿??? INTRODUCCIÓN UNA DE LAS CONSECUENCIAS DE LA
TÍTULO ¿??? INTRODUCCIÓN UNA DE LAS CONSECUENCIAS DE LAKARTA ZGŁOSZENIA UDZIAŁU ZESPOŁU W URSYNÓW MUSIC FEST
 NAME DATE LA – JONES PERIOD
NAME DATE LA – JONES PERIOD