INTERPRETING DATA FROM SOLUBILITY CURVES NAME DATE DIRECTIONS USE
1ST MACAO INTERPRETING CONTEST (EC) 1ST MACAO INTERPRETING CONTEST4E INTERPRETING THE YINTERCEPT AND SLOPE YINTERCEPT THE AMOUNT
CLARIFYING INFORMATION FOR INTERPRETING AIR QUALITY TRENDS AIR
DAGMARA K KUBISIAK DBA ELITE INTERPRETING & TRANSLATION LLC
DUBLIN RAPE CRISIS CENTRE INTERPRETING SENSITIVELY WITH REFUGEES AND
GRADE 7 INFERRING TASK INTERPRETING MESSAGES FROM A MEDIA
INTERPRETING DATA FROM SOLUBILITY CURVES
INTERPRETING DATA FROM SOLUBILITY CURVES
NAME____________________________________
DATE_________________
DIRECTIONS:
Use the handout graph to solv the following problems.
___________1) What is the solubility of
potassium nitrate in 100 grams of water at 100 °C
?
___________2) What is the solubility of potassium
chloride in 100 grams of water at 50 °C ?
___________3
)What is the solubility of sodium chloride in 100 grams of water at
90 °C ?
___________4) What is the minimum temperature
needed to dissolve 180 grams of potassium nitrate in 100 grams of
water
___________5) What is the minimum temperature needed
to dissolve 35 grams of potassium chloride in 100 grams of
water
___________6) At what temperature do potassium
chloride and potassium nitrate have the same solubility
?
___________7 )At what temperature do potassium nitrate
and sodium chloride have the same solubility ?
___________8)
If 110 grams of potassium chloride are mixed with 100 grams of water
at 20 °C, how much will not dissolve
?
___________9) If 250 grams of potassium nitrate are
mixed with 100 grams of water at 85 °C, how much will not
dissolve ?
___________10) If 50 grams of sodium chloride
are mixed with 100 grams of water at 80 °C, how much will not
dissolve ?
___________11) If 15 grams of potassium
chloride are added to 100 grams of water at 30 °C, how much more
must be added to saturate the solution?
___________12) If
170 grams of potassium nitrate are added to 100 grams of water at 80
°C, how much more must be added to saturate the solution
?
___________13) If 10 grams of sodium chloride are added
to 100 grams of water at 100 °C, how much more must be added to
saturate the solution ?
___________14) 100 grams of water
are saturated with potassium chloride at 35 °C. If this solution
is heated to 95oC, how much more can be dissolved ?
___________15)
100 grams of water are saturated with potassium nitrate at 20 °C.
If the solution is heated to 80oC, how much more can be
dissolved?
___________16) 100 grams of water at 50 °C
are saturated with sodium chloride. If this solution is heated to 100
°C, how much more can be dissolved ?
___________17)
100 grams of water at 95 °C are saturated with potassium nitrate.
If this solution is cooled to 35oC, how much of the solid will
precipitate(change from the dissolved state to the solid state)
?
___________18) 100 grams of water at 90 °C are
saturated with potassium chloride. If this solution is cooled to
35oC, how much of the solid will precipitate?
___________19)
How much potassium nitrate will dissolve in 50
grams of water at 95 °C ?
___________20) How much
potassium nitrate will dissolve in 10 grams of
water at 10 °C ?
___________21) How much
potassium chloride will dissolve in 50 grams of
water at 50 °C ?
___________22) How much
potassium chloride will dissolve in 25 grams of
water at 80 °C ?
___________23) If 50 grams of
water are saturated at 90 °C with potassium nitrate and then
cooled to 40°C, how much will precipitate ?
___________24)
What temperature is needed to dissolve twice as much potassium
chloride as can be dissolved at 0 °C in 100 grams of water
?
___________25) What temperature is needed to dissolve
twice as much potassium nitrate as can be dissolved at 10 °C in
100 grams of water ?
identifying_and_interpreting_theme
INTERPRETING DATA FROM SOLUBILITY CURVES NAME DATE DIRECTIONS USE
INTERPRETING IN MENTAL HEALTH CONTEXTS VIA SKYPE (PRODUCED FOR
Tags: curves name____________________________________, directions, interpreting, solubility, curves
- SENATE NO 2909 STATE OF NEW JERSEY 215TH LEGISLATURE
- NORMATIVA SOBRE ACCESIBILIDAD EN EL ENTORNO URBANO FACCURBAII
- INDICACIÓN DE RADIOGRAFÍA DE TOBILLO INDICACIÓN DE RADIOGRAFÍA DE
- RISK ASSESSMENT FORM CHEMISTRY RESEARCH LABORATORY BEFORE STARTING THE
- WORLD BANK STRATEGY TRANSITIONAL SUPPORT STRATEGY (TSS) TRANSITION
- WYK 2 ŚRODOWISKO ŻYCIA MIKROORGANIZMÓW (CZYNNIKI ABIOTYCZNE) ŚRODOWISKO ŻYCIA
- GOBIERNO DE CANTABRIA CONSEJERÍA DE INNOVACIÓN INDUSTRIA TURISMO Y
- FLASH D’INFORMATION N° 1 JANVIER 2018 CE QUIL FAUT
- 0 ACHIEVING TRINITY 2010 STRATEGIC PLAN 20062010 ACHIEVING TRINITY
- FF 2020 STATUT EDUCA UND REVISION STATUT DER SCHWEIZERISCHEN
- Kontrolni Zadatak – Imenice 1sledeće Imenice Razvrstaj u Tabelu
- MEMORANDUM DATE APRIL 1 2006 TO INVESTIGATORS AND SPONSORS
- SECURE APPLICATION DEVELOPMENT LEAST PRIVILEGE USER ACCOUNT CONTROL AND
- DELIBERAZIONE N 55 DEL 11042007 PAGINA 13 DI
- EUROPEAN CHARTER FOR RURAL PRACTICE A EUROPEAN CHARTER
- EL REPARTO DE AFRICA IMPORTANCIA ESTRATÉGICA ESPAÑA Y PORTUGAL
- SCCR46 PÁGINA 13 OMPI S SCCR46 ORIGINAL INGLÉS FECHA
- POST TITLE (THIS FIELD MUST BE COMPLETED) SCHOOL
- MEJORA DE LA COMPETENCIA BÁSICA EN LECTURA PARA PRIMARIA
- K VITTERING FOR AFLEVERING AF FARLIGT AFFALD DRIVER
- ISBN Y DL EN TRÁMITE GRUPO COMPETENCIAS UNIVERSIDAD
- PROGRAM STUDI TEKNIK INFORMATIKA NAMA ………………………… SEKOLAH TEKNIK ELEKTRO
- ATHENEE ROYAL RUE DE NOAILLES 3 6460 CHIMAY DIRECTION
- (ALLEGATO N 2) VALUTAZIONE DEL COMPORTAMENTO INDICATORI RELAZIONE CON
- !DOCTYPE HTML HTML LANGPL HEAD META CHARSETUTF8 TITLESZPZP26120419
- AUTOMOTIVE GLASS DEALERS REPAIRERS SALVAGE VEHICLES IN RESPONSE TO
- WILLIAM JEWELL COLLEGE SALARY REDUCTION AGREEMENT 1 PARTICIPANT INFORMATION
- CARTAS DE LOS LECTORES → DIRIGIDAS AL DIRECTOR DE
- OFFICIAL SENSITIVE – WHEN COMPLETE PERSONAL REFERENCE REQUEST I
- MODULE 16 ACTIVITY 161 SCENARIO — DIFFERENTIATING DOCUMENTS FROM
 HIDROCARBUROS AROMÁTICOS 1 ESTRUCTURA NOMENCLATURA Y PROPIEDADES FÍSICAS
HIDROCARBUROS AROMÁTICOS 1 ESTRUCTURA NOMENCLATURA Y PROPIEDADES FÍSICASTEMELJEM ČLANKA 30I ČLANKA 133 STAVAK 3 ZAKONA O
AP ART HISTORY EXAM REVIEW 74 PART 1 ANCIENT
THE IRS REQUIRES VISA PURCHASES TO BE DOCUMENTED AND
OTROS MODELO DE QUEJA PARA DENUNCIAR UNA DISCRIMINACIÓN ANTE
 CICLO MÚSICA DE CÁMARA AUDITORIO DE GALICIA (SALA MOZART)
CICLO MÚSICA DE CÁMARA AUDITORIO DE GALICIA (SALA MOZART)UN GUIÓN LITERARIO INÉDITO DE MARÍA TERESA LEÓN GABRIELE
ZADANIE NR 4 – WARZYWA OWOCE KONSERWOWE LP ASORTYMENT
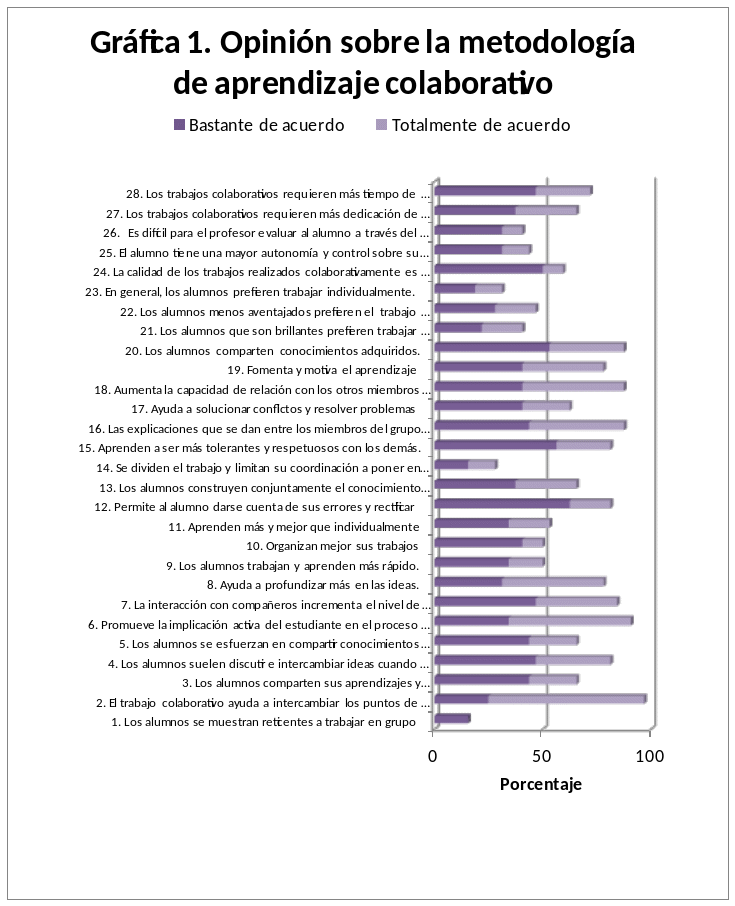 QUE PIENSAN LOS PROFESORES Y ALUMNOS SOBRE LA METODOLOGÍA
QUE PIENSAN LOS PROFESORES Y ALUMNOS SOBRE LA METODOLOGÍA5 CONSIGLIO COMUNALE CONGIUNTO CATANZAROLAMEZIA TERME INTANTO CONSENTITEMI DI
 MODULE – SECTION 8 – INSTALLATION D’UN ÉLECTROFILTRE 1
MODULE – SECTION 8 – INSTALLATION D’UN ÉLECTROFILTRE 1 2020 WICT MENTORING CIRCLES MENTOR APPLICATION THANK YOU FOR
2020 WICT MENTORING CIRCLES MENTOR APPLICATION THANK YOU FORVOORBEELD VAN EEN VERSLAG VAN FEITELIJKE BEVINDINGEN IN VERBAND
KANTİN İŞLETMECİSİ MESLEK ALANI MILLI EĞITIM BAKANLIĞI ÇIRAKLIK VE
 ATLETSKA STAZA MLADOST OPIS ZAHVATA INVESTICIJA OBUHVAĆA SANACIJU ATLETSKE
ATLETSKA STAZA MLADOST OPIS ZAHVATA INVESTICIJA OBUHVAĆA SANACIJU ATLETSKE URWERK PRESENTA EL UR202 TURBINA AUTOMATICA GINEBRA ABRIL 2008
URWERK PRESENTA EL UR202 TURBINA AUTOMATICA GINEBRA ABRIL 2008GAINWELL TECHNOLOGIES CLAIMS DEPARTMENT CLAIM TYPE CLAIM SUBMISSIONS GAINWELL
JMÉNO PŘÍJMENÍ ŽÁKA ŽÁKYNĚ TŘÍDY ADRESA V…………… DNE ………………
EN NOTA SUSCRITA POR REPRESENTANTES CON FIRMA REGISTRADA EN
 SAMBUTAN KETUA PENGADILAN TINGGI BANJARMASIN (DRHJMARNI EMMY MUSTAFA SHMH
SAMBUTAN KETUA PENGADILAN TINGGI BANJARMASIN (DRHJMARNI EMMY MUSTAFA SHMH