CONDUCTIVITY OF SOLUTIONS THE EFFECT OF CONCENTRATION CONDUCTIVITY OF
21 CONTROLLING THE CONDUCTIVITY IN OXIDE SEMICONDUCTORS A32 CONDUCTIVITY OF SOLUTIONS AND MOLTEN SALTS DESCRIPTION A
33 CONDUCTIVITY AS AN ENDPOINT INDICATOR DESCRIPTION A LIGHT
4 THERMATEST MODEL TCFCM COMPARATIVE INSTRUMENT THERMAL CONDUCTIVITYTHE RATE
5 SUPERCONDUCTIVITY BASIC PHENOMENON IF A MATERIAL IS
CONDUCTIVITY LAB C12507 INTRODUCTION AT SWIMMING POOLS SWIMMERS MUST
14 Conductivity Solution
Conductivity of Solutions: The Effect of Concentration
Conductivity
of Solutions:
The Effect of
Concentration
If an ionic compound is dissolved in water, it dissociates into ions and the resulting solution will conduct electricity. Dissolving solid sodium chloride in water releases ions according to the equation:
NaCl(s)
![]() Na+(aq) + Cl–(aq)
Na+(aq) + Cl–(aq)
In this experiment, you will study the effect of increasing the concentration of an ionic compound on conductivity. Conductivity will be measured as concentration of the solution is gradually increased by the addition of concentrated NaCl drops. The same procedure will be used to investigate the effect of adding solutions with the same concentration (1.0 M), but different numbers of ions in their formulas: aluminum chloride, AlCl3, and calcium chloride, CaCl2. A computer-interfaced Conductivity Probe will be used to measure conductivity of the solution. Conductivity is measured in microsiemens per centimeter (µS/cm).
OBJECTIVES
In this experiment, you will
Use a Conductivity Probe to measure the conductivity of solutions.
Investigate the relationship between the conductivity and concentration of a solution.
Investigate the conductivity of solutions resulting from compounds that dissociate to produce different number of ions.
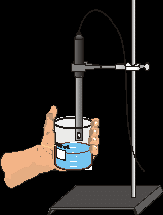
MATERIALS
|
computer |
distilled water |
|
Vernier computer interface |
100 mL beaker |
|
Logger Pro |
1.0 M NaCl solution |
|
Vernier Conductivity Probe |
1.0 M CaCl2 solution |
|
ring stand |
1.0 M AlCl3 solution |
|
utility clamp |
stirring rod |
|
wash bottle |
tissue |
PROCEDURE
1. Obtain and wear goggles.
2. Your experiment setup should look like Figure 1. The Conductivity Probe is already attached to the interface. It should be set on the 0–2000 µS/cm position..
3. The file named “14 Conductivity Solutions” from the Chemistry with Vernier folder, should already be open on your computer
4. Add 70 mL of distilled water to a clean 100 mL beaker. Obtain a dropper bottle that contains 1.0 M NaCl solution.
5. Before adding any drops of solution:
Click
![]() .
.
Carefully raise the beaker and its contents up around the Conductivity Probe until the hole near the probe end is completely submerged in the solution being tested. Important: Since the two electrodes are positioned on either side of the hole, this part of the probe must be completely submerged.
Monitor the conductivity of the distilled water until the conductivity reading stabilizes.
Click
![]() ,
and then lower the beaker away from the probe. Type 0 in the
edit box (for 0 drops added). Press the ENTER
key to store this data pair. This gives the conductivity of the
water before any salt solution is added.
,
and then lower the beaker away from the probe. Type 0 in the
edit box (for 0 drops added). Press the ENTER
key to store this data pair. This gives the conductivity of the
water before any salt solution is added.
6. You are now ready to begin adding salt solution.
Add 1 drop of NaCl solution to the distilled water. Swirl the solution to ensure thorough mixing.
Raise the beaker until the hole near the probe end is completely submerged in the solution. Swirl the solution briefly.
Monitor the conductivity of the solution until the reading stabilizes.
Click
![]() ,
and then lower the beaker away from the probe. Type 1 (the
total drops added) in the edit box and press ENTER.
,
and then lower the beaker away from the probe. Type 1 (the
total drops added) in the edit box and press ENTER.
7. Repeat the Step 6 procedure, entering 2 this time.
8. Continue this procedure, adding 1-drop portions of NaCl solution, measuring conductivity, and entering the total number of drops added—until a total of 8 drops have been added.
9. Click
![]() when you have finished collecting data. Dispose of the beaker
contents as directed by your teacher. Rinse the probe tip with
distilled water from a wash bottle. Carefully blot the probe dry with
a tissue.
when you have finished collecting data. Dispose of the beaker
contents as directed by your teacher. Rinse the probe tip with
distilled water from a wash bottle. Carefully blot the probe dry with
a tissue.
10. Prepare the computer for data collection. From the Experiment menu, choose Store Latest Run. This stores the data so it can be used later, but it will be still be displayed while you do your second and third trials.
11. Repeat Steps 4–10, this time using 1.0 M AlCl3 solution in place of 1.0 M NaCl solution.
12. Repeat Steps 4–9, this time using 1.0 M CaCl2 solution.

 13. Click
on the Linear Fit button,
13. Click
on the Linear Fit button,
![]() .
Be sure all three data runs are selected, then click
.
Be sure all three data runs are selected, then click
![]() .
A best-fit linear regression line will be shown for each of your
three runs. In your data table, record the value of the slope,
m, for each of the three
solutions. (The linear regression statistics are displayed in
a floating box for each of the data sets.)
.
A best-fit linear regression line will be shown for each of your
three runs. In your data table, record the value of the slope,
m, for each of the three
solutions. (The linear regression statistics are displayed in
a floating box for each of the data sets.)
14. To print a graph of concentration vs. volume showing all three data runs:
Label all three curves by choosing Text Annotation from the Insert menu, and typing “sodium chloride” (or “aluminum chloride”, or “calcium chloride”) in the edit box. Then drag each box to a position near its respective curve.
Print a copy of the graph, with all three data sets and the regression lines displayed. Enter your name(s) and the number of copies of the graph you want.
Name - ________________________________
partner - _____________________________
partner - _____________________________
DATA and observations
|
Solution |
Slope, m |
|
1.0 M NaCl |
|
|
1.0 M CaCl2 |
|
|
1.0 M AlCl3 |
|
PROCESSING THE DATA
Describe the appearance of each of the three curves on your graph.
Describe the change in conductivity as the concentration of the NaCl solution was increased by the addition of NaCl drops.
The initial concentration of each salt solution was 1.0M. Calculate the final concentration of the salt solution after the addition of the last drop of solution to the 70mL of water. 8 drops = 0.8mL
Write a chemical equation for the dissociation of NaCl, CaCl2, and AlCl3 in water.
Calculate the final concentration of each ion and the total ion concentration in:
the NaCl solution:
the CaCl2 solution:
the AlCl3 solution:
Which graph had the largest slope value? ____________ The smallest slope? ______________ Since all solutions had the same original concentration (1.0 M), what accounts for the difference in the slope of the three plots? Explain.
Conclusion:
State the results of the lab. State the relationship between conductivity and ion concentration.
Chemistry with
Vernier 14 -
CONDUCTIVITY MEASUREMENT IN ΜFLUIDIC POLYMER CHIP BACKGROUND CONDUCTIVITY
CONDUCTIVITY METER BUILDING AND CALIBRATION INSTRUCTIONS MATERIALS LIST
CONDUCTIVITY OF ELECTROLYTES AIM T HE AIM OF THIS
Tags: conductivity of, between conductivity, conductivity, solutions, concentration, effect
- POŠTOVNÍ KURÝR PŘÍPRAVA A ZAČÁTEK HRY KAŽDÝ
- SEMINARIO REGIONAL “AUTOREFORMA SINDICAL Y TRABAJADORASES EN LA ECONOMIA
- TŰZVÉDELMI MŰSZAKI IRÁNYELV TVMI TŰZOLTÓ KAPCSOK TARTALOMJEGYZÉK 1 ALKALMAZÁSI
- CARACTERÍSTICAS SUPERFICIALES DEL PAVIMENTO RÍGIDO DEL AEROPUERTO INTERNACIONAL“ABEL
- EKSAMEN MASTER I RETTSSOSIOLOGI OG KRIMINOLOGI VÅR 2006
- 29 REFERENDUM CONSULTIVO REGIONAL Y REFORMA CONSTITUCIONAL EL CASO
- PROJECT MANAGEMENT – INDIVIDUAL REPORT STEP DESCRIPTION OTHER
- ACTIVIDADES SEMANAL PRÁCTICAS DEL LENGUAJE SEMANA DEL 64 AL
- BŪVDARBU LĪGUMS 20 GADA VIENOTAIS
- BASES QUE HAN DE REGIR EL PROCÉS SELECTIU PER
- CRNA GORA GLAVNI GRAD PODGORICA SEKRETARIJAT ZA PLANIRANJE PROSTORA
- 20211130 BIBLIOGRÁFIA 21 V AJA NAGYKÖZSÉG (HONLAP ALAPJÁN) VAJA
- UREDBA O DAVČNI OBRAVNAVI POVRAČIL STROŠKOV IN DRUGIH DOHODKOV
- TEMA 65 DERECHO CIVIL 1 INEFICACIA DE LOS CONTRATOS
- APPLICATION TO CONDUCT RESEARCH AT A SIGNIFICANT PALAEONTOLOGICAL SITE
- PRÍLOHA Č 27 K VYHLÁŠKE Č 252004 Z
- TEACHER EDUCATION FOR INCLUSION PROFILE OF INCLUSIVE TEACHERS
- ATTACHMENT 2 CHANGE ORDER 4 BLANKET CONTRACT B90451 42102
- NJOFTIM PËR KONTRATË PUNË NË PËRPUTHJE ME NENIN 5
- LABORATORIOS Y TALLERES PLANTELES DE CECYTE PLANTEL I “LOMAS
- HOW TO MAKE AN APPOINTMENT WITH YOUR DOCTOR WHO
- ÁROP1A2A20080167 PROJEKT IRÁNYÍTÁSI ÉS DÖNTÉSHOZATALI RENDJÉNEK BEMUTATÁSA PROJEKTGAZDA BEMUTATÁSA
- AUTORITZACIÓ PER A LA UTILITZACIÓ D’INFORMACIÓ TRIBUTÀRIA PER A
- [LOCAL AUTHORITY] APPENDIX D – TEMPLATE AND GUIDANCE DBOM
- CAPÍTULO 5 512 EJERCICIOS RESUELTOS PARA LA REPETICIÓN DO…WHILE
- AV DE CAMPANAR 32 46015 VALÈNCIA INSTRUCCIONS DE
- FAGLIGT SELSKAB FOR HYGIEJNESYGEPLEJERSKER INVITERER TIL TEMADAG D 23
- PILS LAUKUMS 4 RIGA LV1365 PHONE +371 67228985 FAX
- EVALUACIÓN NACIONAL DEL SISMED 2006 HOSPITALES INDICADOR DE
- 4 BLOCKS ON EMPLOYMENT RIGHTS IN MORROCCAN OCCUPIED
ARBETSFORMER OCH UTDELNINGSPOLICY FÖR DBFS UNDERSTÖDSSTIFTELSE I UPPSALA LÄN
NO 000000 THE STATE OF TEXAS § IN THE
DEKLARACJA PRZYSTĄPIENIA DO POLSKIEGO TOWARZYSTWA PRZESYŁU I ROZDZIAŁU ENERGII
 ESTADÍSTICOS PROFESORES 2000 1 2 3
ESTADÍSTICOS PROFESORES 2000 1 2 3  APRUEBAN ACTUALIZACIÓN DE INFORMACIÓN Y CATASTRO DE TENDIDO DE
APRUEBAN ACTUALIZACIÓN DE INFORMACIÓN Y CATASTRO DE TENDIDO DE1941 M BIRŽELIO 21OJI ŠEŠTADIENIS JAUNAS PUSKARININKIS PAKĖLĖ AKIS
TERMINARZ ROZGRYWEK LIGI KIELCE A1 JUNIOR KLO JUNST GR5
M F NO FORM MF03 FOUNDATIONS ACT 2011 NOTICE
 THE LATINAMERICAN WATER COURT’S JURY CENSORS FOUR LATINAMERICAN COUNTRIES
THE LATINAMERICAN WATER COURT’S JURY CENSORS FOUR LATINAMERICAN COUNTRIESUNIT 3 – DISCUSSING LIVING SITUATIONS UNIT 3 –
 SYMBOLISM IN LORD OF THE FLIES SYMBOLISM PLAYED AN
SYMBOLISM IN LORD OF THE FLIES SYMBOLISM PLAYED AN SVEUČILIŠTE JURJA DOBRILE U PULI IZVJEŠĆE O PROVEDENOJ NEOVISNOJ
SVEUČILIŠTE JURJA DOBRILE U PULI IZVJEŠĆE O PROVEDENOJ NEOVISNOJPLES MANŽELSKÝCH SETKÁNÍ V SOBOTU 29 LEDNA 2005 OD
COTIZACION AL REGIMEN DE AUTÓNOMOS NACIMIENTO Y FIN DE
 L FORMULARIO SOLICITUD LICENCIA FEDERATIVA FEDERAZIOAREN LIZENTZIA ESKATZEKO FORMULARIOA
L FORMULARIO SOLICITUD LICENCIA FEDERATIVA FEDERAZIOAREN LIZENTZIA ESKATZEKO FORMULARIOA THE RULES OF ORIGIN NATIONALITY AND RESTRICTED GOODS 1
THE RULES OF ORIGIN NATIONALITY AND RESTRICTED GOODS 1 ZESZYTY NAUKOWE UNIWERSYTETU SZCZECIŃSKIEGO NR 000 EKONOMICZNE PROBLEMY USŁUG
ZESZYTY NAUKOWE UNIWERSYTETU SZCZECIŃSKIEGO NR 000 EKONOMICZNE PROBLEMY USŁUG DOCTORAL CANDIDATE’S THESIS DECLARATION FORM THIS FORM MUST BE
DOCTORAL CANDIDATE’S THESIS DECLARATION FORM THIS FORM MUST BE9 INTERESSEGRUPPEN FOR PRIVATPRAKTISERENDE PSYKOLOGER EN HISTORISK OVERSIKT
 UNEPCMSCOP12DOC2432 12TH MEETING OF THE CONFERENCE OF THE
UNEPCMSCOP12DOC2432 12TH MEETING OF THE CONFERENCE OF THE