CONFLICT OF INTEREST IN RESEARCH FINANCIAL DISCLOSURE FORM COMPLETE
REGLAMENTO DE RESOLUCIÓN EXTRAJUDICIAL DE CONFLICTOS ARTÍCULO1 SURFACE MATTERS CASE CONFLICT IN FREE RELATIVE CONSTRUCTIONS
14 THE SELF IN CONFLICT WITH ITSELF A HERACLITEAN
2 RESPETO DE LOS DERECHOS HUMANOS EN LOS CONFLICTOS
2 SESSION 10 CLIMATECHANGE POLICIES AND TRADE RULES CONFLICT
20152016 PURPOSE OF THE ADVISORY BOARD THE STUDENT CONFLICT
CONFLICT OF INTEREST IN RESEARCH

CONFLICT OF INTEREST IN RESEARCH
FINANCIAL DISCLOSURE FORM
Complete EITHER Part A or Part B of this form and upload a signed copy in iRIS.
NAME: DEPARTMENT: PHONE:
PROJECT TITLE:
PART A. FINANCIAL CERTIFICATION
I certify that:
1. I, or any member of my family (spouse, domestic partner, children, and any other person living in the same household), have not entered into any financial arrangement with the study sponsor(s) (e.g., bonus, royalty, or other financial incentive) whereby my compensation could be affected by the outcome of the research;
2. I, or any member of my family, do not have a proprietary interest (e.g., patent, trademark, copyright, licensing agreement, etc.) in the product tested in the clinical study;
3. I, or any member of my family, do not have any equity interest (i.e., ownership interest, stock option, or other financial interest whose value cannot be calculated with reference to publicly traded prices or other measure of fair market value) in the study sponsor(s).
4. I, or any member of my family, have not received any payment from the study sponsor(s), or manufacturer of the product or service being tested, having a total value in excess of $0 when aggregated for the immediate family other than payments for conducting the research study. (Examples of such significant payments include, but are not limited to, grants or funding for ongoing research, compensation in the form of equipment, retainers for ongoing consultation and honoraria that are (a) paid directly to investigators or to the institution with which the investigators are affiliated, and (b) paid in support of investigator activities.);
5. I, or any member of my family, do not have a Board or executive relationship related to the research, regardless of compensation.
I have answered fully and to the best of my ability and will update this form promptly if my circumstances change.
__________________________________________ ________________________
Signature
Date
PART B. FINANCIAL DISCLOSURE
I disclose the following (check all that apply and attach detailed information):
___ I, or a member of my family, have entered into financial arrangement(s) with the study sponsor(s) (e.g., bonus, royalty, or other financial incentive) whereby my compensation could be affected by the outcome of the research;
___ I, or a member of my family, have a proprietary interest (e.g., patent, trademark, copyright, licensing agreement, etc.) in the product tested in the clinical study;
___ I, or a member of my family, have an equity interest (i.e., ownership interest, stock option, or other financial interest whose value cannot be calculated with reference to publicly traded prices or other measure of fair market value) in the study sponsor(s);
___ I, or a member of my family, are receiving payments from the study sponsor or research entities other than the sponsor having a total value in excess of $0 when aggregated for the immediate family, other than payments for conducting the research study. (Examples of such payments include, but are not limited to, grants or funding for ongoing research, compensation in the form of equipment, retainers for ongoing consultation and honoraria that are (a) paid directly to investigators or to the institution with which the investigators are affiliated, and (b) paid in support of investigator activities.);
___ I, or a member of my family, have a Board or executive relationship related to the research, regardless of compensation;
___ I am aware of the Trinity Health Of New England policy on financial disclosure regarding amounts between $5,000 and $50,000 indicating I need a management plan and cannot serve as the PI. I am also aware that any amount over the threshold of $50,000 will not allow me to be involved on the study at all. More information on this policy can be found on the infonet (Investigator Conflicts of Interest in Funded Research Policy)
I have answered fully and to the best of my ability and will update this form promptly if my circumstances change.
__________________________________________ ________________________
Signature Date
Financial Interest Related to Research is defined as a financial interest in the sponsor, product or service being tested, or competitor of the sponsor. This covers anything of monetary value, including, but not limited to, salary or other payments for services (e.g., consulting fees or honoraria); equity interest (e.g., stocks, stock options or other ownership interest); and intellectual property rights (e.g., patents, copyrights and royalties from such rights). For the purposes of this policy, Disclosable Financial Arrangements are:
Compensation made to the investigator in which the value of compensation could be affected by study outcome.
A proprietary interest in the tested product, including but not limited to: a patent, trademark, copyright or licensing agreement.
Any equity interest in the sponsor of a covered study, i.e., any ownership interest, stock options, or other financial interest whose value cannot be readily determined through reference to public prices.
Any equity interest in a publicly held company that exceeds $0 in value.
Payments of other sorts, which are payments that have a cumulative monetary value of $0 or more made by the sponsor of a covered study to the investigator or the investigator’s institution to support activities of the investigator exclusive of the costs of conducting the clinical study or other clinical studies (e.g., a grant to fund ongoing research, compensation in the form of equipment or retainers for ongoing consultation or honoraria) during the time the clinical investigator is carrying out the study and for 1 year following completion of the study.
The term does not include:
Salary, royalties, or other remuneration from Trinity Health Of New England;
Income from seminars, lectures, or teaching engagements sponsored by public or non-profit entities;
Income from service on advisory committees or review panels for public or non-profit entities;
An equity interest that when aggregated for the Investigator and the Investigator’s family members, meets both of the following tests: Does not exceed $0 in value as determined through reference to public prices or other reasonable measures of fair market value, and does not represent more than 0% ownership interest in any single entity;
Salary, royalties or other payments that when aggregated for the Investigator and the Investigator’s family members over the next twelve months, are not reasonably expected to exceed $0.
Non-Financial Interest Related to Research is defined as an interest in the sponsor, product or service being tested, or competitor of the sponsor which excludes financial interest. For the purposes of this policy, Disclosable Non-Financial Arrangements are:
Having a Board or executive relationship related to the research, regardless of compensation.
Have been involved, or plan to become involved in the design, conduct, or reporting of the research.
July 2017
3 CONFLICT MANAGEMENT ASSIGNMENT ACTUAL NEGOTIATION PAPER INSTRUCTIONS DURING
33 SOME FACTS TO UNDERSTAND THE NORTH MALUKUS CONFLICT
42 STATUS CONFLICT IN NEGOTIATION YERI CHO JENNIFER R
Tags: complete either, financial, interest, research, complete, disclosure, conflict
- “2015” BOB VIGARS SEASON’S OPENER 40TH ANNUAL SATURDAY NOVEMBER
- LA MUSIQUE FRANÇAISE DU XVIIE SIÈCLE FACE À LA
- RESMI GAZETE TARIHI 27122012 RESMI GAZETE SAYISI 28510 SABİT
- NOTA DE PRENSA DOS ESTUDIANTES DE ALMERÍA PODRÁN IR
- NOTICE OF OPENING A TRUST ACCOUNT – CONVEYANCERS
- 16 PROCESO 166IP2012 INTERPRETACIÓN PREJUDICIAL A SOLICITUD
- SCOTTISH QUALIFICATIONS AUTHORITY PROCESS FOR APPEALS ALL AWARDING BODIES
- “DISCIPLINARE PER I CONTROLLI AMBIENTALI” ALLEGATO A ALLA DELIBERAZIONE
- SCTC2018 THE 15TH SPACECRAFT CHARGING TECHNOLOGY CONFERENCE PROGRAMINDEXES PROGRAM
- CLASIFICACIÓN GENERAL POSICIÓN DORSAL TIEMPO PROMEDIO TIEMPO NETO CORREDOR
- AKTIZYM + PERLE VERTE REF 3192B APPLICATIONS
- MEMORYBASED INDEXING REQUIRES NO TEMPORARY FILES MEMORY NEEDED A
- SILABUS MATA PELAJARAN PEKERJAAN DASAR TEKNIK OTOMOTIF (DASAR BIDANG
- HAYAT STANDARDI UYGULAMASI HAYAT STANDARDI ESASININ UYGULAMASINDA DIKKATE ALINACAK
- GRAD KOPRIVNICA ZRINSKI TRG 1I KOPRIVNICA KOJEG ZASTUPA GRADONAČELNIK
- RECTÁNGULO 2 AGENDA DIALOGO DE ACTORES PARA
- SECTION 23 51 00 OR 23 35 00 PART1
- REFERAT FRA GENERALFORSAMLINGEN NORSK ORTOPEDISK FORENING HOLMENKOLLEN PARK HOTELL
- 0 PLAN MOT DISKRIMINERING OCH KRÄNKANDE BEHANDLING FÖR BRÅLANDA
- NATIONAL PLANNING AUTHORITY NOTICE OF EXPRESSION OF INTEREST FOR
- FIELD TRIPS THE DISTRICT HAS DEVELOPED A POLICY
- POSEŁ ADAM SZEJNFELD W 2010 ROKU SPRAWOZDANIE Z DZIAŁALNOŚCI
- ZAKON O IZMJENAMA I DOPUNAMA ZAKONA O ZAŠTITI NA
- BYLAWS DAHLONEGA DOWNTOWN DEVELOPMENT AUTHORITY DAHLONEGA GEORGIA ARTICLE I
- UN PUEBLO DE LA SIERRA DE HUELVA ÉRASE UNA
- MENTORING AND INDUCTION QUOTES “YOU CAN’T MEASURE SUCCESS IF
- CANCIÓN DE LOS DEDOS AB 103 “ESTE DEDICO PIDE
- CONSEJERÍA DE EDUCACIÓN DELEGACIÓN PROVINCIAL DE MÁLAGA CEIP MANZANO
- GUÍA DE EJERCICIOS DE COMPRENSIÓN LECTORA INFERENCIAL LAS PREGUNTAS
- B UKU PEDOMAN AKADEMIK PROGRAM STUDI TEKNIK INFORMATIKA UNIVERSITAS
1) AŞAĞIDA BELIRTILEN GELIR UNSURLARINDAN HANGISINDE GELIRIN ELDE EDILMESI
 TRANSFER OF TECHNOLOGY (TOT) FOR HIGH TEMPERATURE EROSION RESISTANCE
TRANSFER OF TECHNOLOGY (TOT) FOR HIGH TEMPERATURE EROSION RESISTANCE19 FRESH EXPRESSIONS A RESPONSE TO THE COMMITMENT
 PROTOCOLO PARA LA REALIZACION DE ENSAYOS DE VALORACIÓN AGRONÓMICA
PROTOCOLO PARA LA REALIZACION DE ENSAYOS DE VALORACIÓN AGRONÓMICA PAYROLL OFFICE 2021 SUMMER SESSIONS PAY DATES SUMMER SESSION
PAYROLL OFFICE 2021 SUMMER SESSIONS PAY DATES SUMMER SESSIONPLATÓN Y SU DIÁLOGO LA REPÚBLICA PLATÓN DICE QUE
 COLLOQUE 5 LEVALUATION DANS LENSEIGNEMENT SUPERIEUR EVALUATION DU SYSTÈME
COLLOQUE 5 LEVALUATION DANS LENSEIGNEMENT SUPERIEUR EVALUATION DU SYSTÈME23 CHAPTER 3 REGISTRATION OF ELECTORS AND VOTERS SECTION
3 HC SEMINAR 2 THE PEOPLING OF NEW YORK
PROGRAMA DE SEGURIDAD E HIGIENE EMPRESA CONTROL DE PLAGAS
 SUPERVISOR DE ENVASADO – PLANTA MOTUPE NOS ENCONTRAMOS EN
SUPERVISOR DE ENVASADO – PLANTA MOTUPE NOS ENCONTRAMOS ENBOSNIA’S THREEHEADED BEAST SEJDIĆ AND FINCI V BOSNIA AND
 A SOCIACIÓN DE DIABETES DEL PERÚ – ADIPER TELÉFONO
A SOCIACIÓN DE DIABETES DEL PERÚ – ADIPER TELÉFONO LICEO SCIENTIFICO STATALE “G MARCONI” –FOLIGNO– VIA ISOLABELLA N°1
LICEO SCIENTIFICO STATALE “G MARCONI” –FOLIGNO– VIA ISOLABELLA N°1 J OB DESCRIPTON POST TITLE MST CAN THERAPIST (MULTI
J OB DESCRIPTON POST TITLE MST CAN THERAPIST (MULTIREQUIREMENTS FOR ASSOCIATE MEMBERSHIP THE FOLLOWING INFORMATION MUST BE
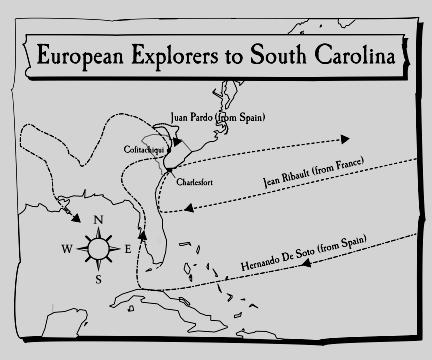 CHAPTER 4 STUDY GUIDE SC HISTORY NAME DATE READ
CHAPTER 4 STUDY GUIDE SC HISTORY NAME DATE READ 311 RELATIONSHIPS AND SEX EDUCATION POLICY POLICY STATEMENT DEFINITION
311 RELATIONSHIPS AND SEX EDUCATION POLICY POLICY STATEMENT DEFINITIONAGREEMENT BETWEEN THE GOVERNMENTS OF THE REPUBLIC OF ANGOLA
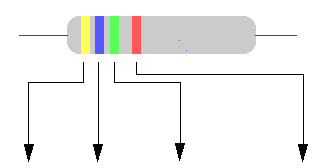 RESISTORES CÓDIGO DE COLORES ES EL CÓDIGO CON EL
RESISTORES CÓDIGO DE COLORES ES EL CÓDIGO CON EL