3 BALANCING REDOX REACTIONS – USING OXIDATION NUMBERS A)
3 BALANCING REDOX REACTIONS – USING OXIDATION NUMBERS A)BALANCING CHEMICAL EQUATIONS CLASS COPY PLEASE DO NOT WRITE
BALANCING CHEMICAL EQUATIONS WORKSHEET 1 H2 + O2 →
BALANCING EQUATIONS NA + NANO3 → NA2O + N2
BALANCING EQUATIONS WORKSHEET 1 ANSWERS BALANCE THE FOLLOWING
BALANCING OXIDATIONREDUCTION EQUATIONS IN THE LABORATORY IT IS IMPORTANT
3
3. Balancing Redox Reactions – Using Oxidation Numbers
a) What are “Oxidation Numbers”?
Sample: K2Cr2O7 K is +1 Cr is +6 O is -2
They are not actual charges!
They are a bookkeeping system used to keep track of an atoms electrons!
i) Rules for Assigning Oxidation Numbers
Oxidation number of an atom as an isolated ion is the charge of the ion.
Eg: Cu2+ has oxidation number of +2
Oxidation number of an atom in elemental form is zero.
Eg. Cu (s) has oxidation number of 0
Eg. O2 (g) has oxidation number of 0
Eg. S8 (s) has oxidation number of 0
Oxidation of common atoms in a compound is its combining capacity
Common atoms include:
Group 1 (+1); Group 2 (+2); Halogens (-1); Oxygen (-2); Aluminum (+3)
The sum of the positive and negative charges in a compound equals zero
Eg. K2Cr2O7
The sum of the positive and negative charges in a polyatomic ion equals the charge of the ion.
Eg. MnO4-
ii) Example: What are the oxidation numbers for all the atoms in Al(NO3)3?
iii) Example: What are the oxidation numbers for each atom in ClO4-? (Tricky!)
iv) Example: What are the oxidation numbers for each atom in KH2PO4?
v) Example: Use changes in oxidation number to identify which species is oxidized and
which is reduced:
Cu (s) + 2AgNO3 (aq) Cu(NO3)2 (aq) + 2Ag (s)
vi) Example: Identify the oxidizing agent and the reducing agent:
3H2S (g) + 2HNO3 (aq) 3S (s) + 2NO (g) + 4H2O (l)
Do Questions: #3-6 page 194-195; #20-23 page 204
b) Balance Redox Using Oxidation Numbers (optional)
i) Procedure:
Separate redox into its two half reactions
Assign oxidation numbers to all atoms that change oxidation number
(not oxygen or hydrogen)
Find change in oxidation number (ON) for each half reaction
Multiply half reactions by a factor to make the overall ON zero when added.
Add the two half reactions together
Balance the O’s by adding H2O and balance the H’s by adding H+
ii) Balance: Cr2O72- + Fe+2 Cr+3 + Fe+3 (acidic)
1st Separate half reactions and 2nd Assign oxidation numbers and 3rd Find ON
4th Make overall ON zero and 5th Add together
6th Balance O’s and H’s
Do Questions: #25 page 210
CALCULATION OF JOINT MOMENTS AND MUSCLE FORCES TRY BALANCING
CASHIER BALANCING (506051) POLICY ALL TRANSACTIONS BY BUSINESS OFFICE
D&C JRCJC SECTION 232113 COMBINATION BALANCING VALVE AND FLOW
Tags: balancing redox, numbers, balancing, reactions, using, redox, oxidation
- OGASUN BATZORDEA COMISIÓN DE HACIENDA IJENTEA 1 TEL
- DETALJPLAN FÖR DNR KU 2011160 FASTIGHETERNA HANÅSA 174 MFL
- SECRETARIA MUNICIPAL DA SAÚDE COORDENAÇÃO DE VIGILÂNCIA EM SAÚDE
- WHY A NATIONAL ENVIRONMENTAL METHODS INDEX IS
- Acta de Absolucion de Observaciones Adjudicacion Directa Selectiva nº
- PERSONA INTERESADA NIF APELLIDOS Y NOMBRE O RAZÓN SOCIAL
- CHORDATA REPTILIA PHYLUM CHORDATA SUB PHYLUM
- VARIANCEDISPERSION TABLES OF VALUES AND GRAPHS OF THE PMF’S
- VERANSTALTUNGSHINWEIS DER ORING AKADEMIE® DER UNABHÄNGIGE HERSTELLER FÜR ORINGE
- ZAŁĄCZNIK NR 6 DO ZARZĄDZENIA NR 151011 …………………………… (MIEJSCOWOŚĆ
- PERF14B ENCUESTA VALORACIÓN PLAN CANARIO DE FORMACIÓN
- MY WEBSITE WILL BE AN ECOMMERCE WEBSITE I WILL
- 10 VERGLEICH VERSCHIEDENER ÖFFENTLICHER ORGANISATIONSFORMEN FÜR NATUR UND LANDSCHAFT
- OFICINA PRINCIPAL PLAZA DE LOS FUEROS Nº 3 TFNO
- CO NOWEGO NA BOISKACH I W SZATNIACH CZYLI SŁÓW
- SPRAWOZDANIE Z MAŁOPOLSKIEJ KONFERENCJI PIELĘGNIAREK I POŁOŻNYCH HOTEL GALAXY
- PLANNING GUIDE FOR EFFECTIVE ROTARY CLUBS APRIL 2012
- TOPIC TEST TOPIC 2 2D ARRAYS MULTIPLE CHOICE
- INCLUDEPICTURE HTTPWWWFIZYKAAMUEDUPLIMAGESLOGOUAMNIEBJPG MERGEFORMATINET ZGŁOSZENIE KANDYDATA NA MIĘDZYNARODOWĄ LETNIĄ
- KLASA 5 PONIEDZIAŁEK ORTOGRAFICZNE PRZYGODY Z RZ I Ż
- THE STRATEGIC PLAN FOR 2014–2020 FOR THE UNIVERSITY SCHOOL
- DRAFT CCSDS EXPERIMENTAL SPECIFICATION FOR NEXT GENERATION SPACE INTERNET
- DISCURSO DEL PRESIDENTE DE COLOMBIA ANDRÉS PASTRANA ARANGO EN
- EXEMPLE DE NOTATION GENRE PROJET DE RÉHABILITATION DES PUITS
- PLIEGOS DE PRESCRIPCIONES TÉCNICAS PARA LA ADQUISICIÓN DE IMPLANTES
- PROTOCOL FOR POLITICAL REPRESENTATIVES OR CANDIDATES VISITING QUEENSLAND RAIL
- III CONCURSO NACIONAL DE PINTURA CIUDAD DE FRÍAS Y
- ADMINISTRACIÓN FEDERAL DE INGRESOS PÚBLICOS IMPUESTOS RESOLUCIÓN GENERAL 2681
- 1 ST ANNUAL CALIFORNIA STEM SYMPOSIUM INVEST IN CALIFORNIA
- INDICE PRESENTACIÓN 5 CAMBIAR LA RELACIÓN CON EL LIBRO
 FUTTERTIERLISTE NUR FÜR MITGLIEDER! ZUBEHÖR AUF ANFRAGE! EINHEITEN PREIS
FUTTERTIERLISTE NUR FÜR MITGLIEDER! ZUBEHÖR AUF ANFRAGE! EINHEITEN PREIS CUADRO DE PERMISOS ADMINISTRACIÓN FORAL DE NAVARRA NAFARROAKO
CUADRO DE PERMISOS ADMINISTRACIÓN FORAL DE NAVARRA NAFARROAKO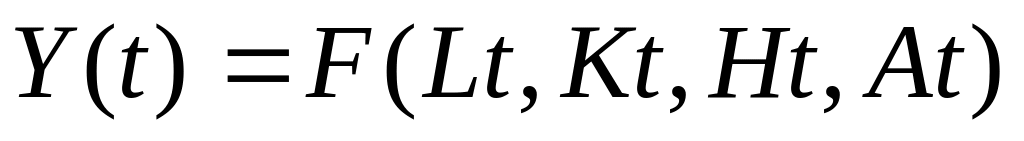 DIFUSIÓN TECNOLÓGICA EN ENTORNOS RESTRINGIDOS ESTIMACIÓN EMPÍRICA PARA
DIFUSIÓN TECNOLÓGICA EN ENTORNOS RESTRINGIDOS ESTIMACIÓN EMPÍRICA PARAMILJÖSAMVERKAN VÄSTRA GÖTALAND VINDKRAFTVERK HANDLEDNING FÖR KOMMUNERNA JUNI 2009
CERTIFICADO DE AUTOCONTROL CTE REFERENCIA Nº EMPRESA PÁGINAVALOR ÍNDICE
VZOREC POGODBE OBČINA ROGATEC POT K RIBNIKU 4 3252
 QUESTIONNAIRE P1 MONTENEGRO STATISTICAL OFFICE LAW ON OFFICIAL STATISTICS
QUESTIONNAIRE P1 MONTENEGRO STATISTICAL OFFICE LAW ON OFFICIAL STATISTICSTRANSCRIPCIÓN FONÉTICA DEL CHISTEWAV [KEˈɹIÐ̞O ˈIXO̥↓‖ TESˈKɹIΒ̞O E̯STAS̬
LA NOCHE DE LOS MUSEOS CARTAGENA 15 DE MAYO
 LABORATORY AVERA SACRED HEART HOSPITAL 501 SUMMIT • YANKTON
LABORATORY AVERA SACRED HEART HOSPITAL 501 SUMMIT • YANKTONSCENARIUSZ ZAJĘĆ NA INTERDYSCYPLINARNEJ ŚCIEŻCE DYDAKTYCZNEJ W OROŃSKU AUTORZY
ACTO EN HOMENAJE A LAS VÍCTIMAS DEL TERRORISMO KURSAAL
 STATE OF OREGON OREGON YOUTH AUTHORITY OYA FOSTER PARENTS
STATE OF OREGON OREGON YOUTH AUTHORITY OYA FOSTER PARENTS JURJEVANJE V BELI KRAJINI PREDSTAVITEV PROMOCIJSKIH PAKETOV 2016 I
JURJEVANJE V BELI KRAJINI PREDSTAVITEV PROMOCIJSKIH PAKETOV 2016 IACTA DE CONSTITUCION APROBACIÓN DE ESTATUTOS ELECCION DE DIGNATARIOS
 PROCÈSVERBAL ASSEMBLÉE GÉNÉRALE ORDINAIRE APRÈSGE – 21 MAI
PROCÈSVERBAL ASSEMBLÉE GÉNÉRALE ORDINAIRE APRÈSGE – 21 MAIADALIMUMAB BIOSIMILARS IN EUROPE AN OVERVIEW 12SALVATORE BELLINVIA 34JR
OTROS MUNDOS OTROS DIOSES MOHS MAYO COMPILADOR OTROS MUNDOS
Terms & Conditions 1 all Bookings Will Require Room
 MAPAS QUE RELATAN HISTORIAS FOLCLOR Y GEOGRAFÍA UNA ACTIVIDAD
MAPAS QUE RELATAN HISTORIAS FOLCLOR Y GEOGRAFÍA UNA ACTIVIDAD