QUESTIONS ON COORDINATION CHEMISTRY 1SKETCH THE STRUCTURES OF ISOMERS
20XX WRITTEN QUESTIONS ON APPLICATION GUIDELINES AS WEALCOHOL SCREENING TOOL THESE TEN QUESTIONS ARE TAKEN
ANSWER THE FOLLOWING QUESTIONS IN SUPPLIED “ANSWER SHEETS”
COVID19 VACCINATIONS FREQUENTLY ASKED QUESTIONS ABOUT THE
DISORDERS OF LEUKOCYTES QUESTIONS ASPHO BOARD REVIEW
FREQUENTLY ASKED QUESTIONS REGARDING SERVICE ACADEMY NOMINATIONS
Table 2
Questions on coordination chemistry
1-Sketch the structures of isomers Co(en)33+ complex ion to show that they are mirror images of each other.
2- What are the coordination number and the oxidation state, respectively, of the cobalt atom in the compound [Co(NH3)5Cl]Cl2?
3- Coordination compounds which contain cyanide, CN-, ligands tend to be yellow whereas coordination compounds which contain water, H2O, ligands tend to be blue or. Explain why?
4-Consider the coordination compound, Na3[Co(CN)6]. Would you expect this compound to be diamagnetic or paramagnetic? Explain your reasoning assuming that the CN- ligand is a strong-field ligand.
5-What are the oxidation number of the iron atom, coordination number and geometric shape, respectively, for the complex ion, [Fe(CN)6]4-?
6- For the complex ion, [Co(en)2(OH2)CN]2+, what are the coordination number (C.N.) and the oxidation number (O.N.) of the central metal atom?
7- The hexaquo manganese(II) ion contains five unpaired electrons, while the hexacyano- ion contains only one unpaired electron. Explain, using Crystal Field Theory.
8- 5) Give an example of each of the following:
Metal Chelate
Low spin complex
High spin complex
9- ) Write down the systematic name for each of the following complexes and indicate the coordination number, oxidation state, of the central ion.
K4[Mn(CN)6]
[CoCl(NH3)5]Cl2
[NiCl(NH3)(en)2]Cl
[Cu(NH3)4(H2O)]SO4
10- Co in [CoF6]3- is a high spin complex. Show the distribution of d electrons for Co3+ in the octahedral d energy level diagram.
11- Using crystal field theory, draw the crystal field d orbital energy level diagram for each of the following complexes by assigning electrons to 3d orbitals.
[Co(en)2]2+ (square planar)
[FeF6]3- (octahedral)
12- The crystal field splitting energy affects the d-orbital occupancy and the magnetic properties of the complex. Explain with example.
13- What is the relationship between the following two complex ions?
I. [Co(NH3)4(NO2)Cl]+ II. [Co(NH3)4(ONO)Cl]+
14-Calculate the crystal field stabilization energy for the high-spin complex Fe(H2O)63+.
15- Which of the following compounds would be paramagnetic?
Sc(NH3)63+ (high spin)
Zn(OH)42- (tetrahedral)
Co(NH3)63+ (high spin)
Fe(CN)64- (low spin)
-Mark Questions
1. Write the IUPAC name of each of the following complexes. (1 mark each)
(a) [CrBr(H2O)5]SO4
(b) [Co Cl (en)2 (ONO)] NO3
(c) [Fe(C2O4)3]3-
(d) [Cr(H2O)5(SCN)]Cl2
(e) Na3[Cr F4(OH)2]
(f) [Pt(NH3)4][PtIICl6]
(g) K4[Fe(CN) ]
h. [RhCl(PPh3)3]
(i) [Ni(CO)4]
(j) [CoCl(en)2(NH3)]2+
(k) [CrCl2(OX)2]3-
(l) K3[Fe(C2O4)3]
(m) [Pt Cl (NH2CH3) (NH3)2]NO2
n. [CoIIICl(NH3)4(NO2)] [Au(CN)2]
(o) K2[HgI4]
(p) Xe[PtF6]
2. Using IUPAC norms, write the formulae for the following: (1 mark each)
(a) potassiumtetrachloropalladate(II)
(b) pentaamminenitrito-O-cobalt(III)chloride
(c) tetraaquadichlorochromium(III)chloridedihydrate
(d) bis(ethylenediamine)nitrito-N-thiocyanato-S-cobalt(III)ion
(e) hexaamminechromium(III)sulphate
(f) pentaamminenitrito-N-cobalt (III) chloride
(g) tetrachlorocuprate(II)
h. potassiumtetracyanonickelate(II)
(i) potassiumhexacyanocobaltate(III)
(j) bis(ethylenediamine)nitrito-N-thiocyanato-S-cobalt(III) ion
(k) tetraaquadichlorochromium(III)chloridedihydrate.
3. The equilibrium constants of [Cu(NH3)4]2+ and [Co(NH3)6]3+ are 1.0 x 10-12 and
6.2 x 10-36 respectively. Which compound is more stable and why?
4. Give one use of Ziegler catalyst.
5. Which of these cannot act as a ligand:NH3, H2O, CO, CH4
6. (a) Write the IUPAC name of the ionisation isomer of [Ni(NH3)5(NO3)]Cl. (1 mark each)
(b) Write the IUPAC name of the linkage isomer of [Co(NH3)5(SCN)] Cl2
(c) Write the IUPAC name of the coordination isomer of [ PtII(NH3)4 ] [ CuCl4 ]
7. What is meant by ‘denticity’?
8. Compare the molar conductivities of the following complexes:
[Pt(NH3)2Cl2] and [Pt(NH3)4][PtIICl6]
9. Name the type of isomerism exhibited by the following isomers:
[PtII(NH3)4][PtCl6] and [PtIV(NH3)4Cl2][PtCl4]
2-Mark Questions
10. Mention four applications of coordination complexes.
11. How will you distinguish [Co(NH3)5 (SO4)] Br and [CoBr(NH3)5] SO4
What kind of isomerism do they exhibit?
12. Which among [Ag(NH3)2]Cl, [Ni(CN)4]2-, [NiCl4]2-
(i) has square planar geometry and (ii) remains colourless in aqueous solution.
13. Using valence bond approach, explain the shape and magnetic behaviour of [Fe(CN)6]3-.
14. Write all isomers of Na[Co(en)2( ox)2]
15. What do the terms ‘inner octahedral’ and ‘outer octahedral’ mean?
16. Explain how [ PtCl2(NH3)2 ] and [ Pt(NH3)6 ] Cl4 will differ in their electrolytic conductance. Give the hybridization and oxidation states of Pt in these complexes.
17. Draw the structures of each of the following:
(a) [ Cr(C2O4)3 ] 3-
(b) [Fe(CO)5]
18. Draw a figure to show the splitting of degenerate d-orbitals in an octahedral crystal field. How does the magnitude of ?o decide the actual configuration of d-orbitals in a complex entity?
19. Discuss the nature of bonding in [Ni(CO)4]
20. What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper(II) sulphate? Why is it that no precipitate of copper sulphide obtained when H2S is passed through this solution?
21. Using valence bond approach, predict the shape of each of the following: (2 mark each)
(a) [CuCl4]2- (paramagnetic)
(b) [Ni(CN)4]2- (diamagnetic)
(c) [Cu(NH3)4]2+ (diamagnetic)
(d) [Ag(NH3)2]+ (diamagnetic)
(e) [Ni(NH3)6]2+ (paramagnetic)
3-Mark Questions
22. Explain the terms:
(a) ambidentate ligand
(b) chelate complexes
(c) coordination number.
23. (a) A coordination compound has the formula CoCl3.4NH3. It does not liberate NH3 but forms a precipitate of AgCl on treatment with AgNO3 solution. Write the structure and IUPAC name of the complex.
(b) Name two properties of the central metal ion which enable it to form stable complex entities.
(c) The formation of complex compounds finds application in the extraction of some metals. Furnish one example to support the above statement
24. Draw the structures of:
(a) cis-dichlorotetracyanochromate(III)
(b) mer-triamminetrichlorocobalt(III)
(c) fac-triaquatrinitrito-N-cobalt(III).
25. Explain the role of coordination compounds in:
(a) medicine
(b) extraction of metals
(c) analytical chemistry
26. Account for the following:
(a) Though CO is a weak Lewis base, yet it forms a number of stable metal carbonyls.
(b) K3[Fe(CN)6] shows very low paramagnetism while K3[FeF6] is highly paramagnetic.
(c) Aqueous silver nitrate forms precipitate with [Co(NH3)6] Cl2 but does not form precipitate with [Pt(NH3)2Cl2].
27. (a) What are the factors on which the stability of a complex depends?
(b) Write the IUPAC nomenclature of the K3[Cr(NO)NH3(CN)4] complex along with its hybridization and structure [K3[Cr(NO)NH3(CN)4], ?=1.73 BM]
(c) With the help of crystal field theory, predict the number of unpaired electrons in [Fe(CN)6]4- and [Fe(H2O)6]2+ complexes
29. (a) Give the IUPAC name of the ionization isomer of [Co(H2O)(NH3)4(NO3)]SO4
(b) Explain the meaning of chelate complexes with an example.
30
(a) Draw the geometrical isomers of [Pt(NH3)4Cl2]2+.
(b) Account for the following:
(i) The complex [Co(en)(NH3)4]Cl3
is more stable than [Co(NH3)6]Cl3.
(ii)
In an octahedral complex eg
set of d-orbitals is raised in energy as compared to t2g
set.
Last modified: Tuesday, 6 November 2007, 11:51 PM
Questions on Transition metals
Give reasons for the following questions related to transition elements:
Transition metals form alloys with other transition metals easily.
Melting points of transition metals increase up to the middle of the series
and then decrease.
Transition metals and their compounds are good catalysts.
Zn, Cd and Hg are softer and volatile metals.
Cr and Cu exhibit exceptional electronic configurations.
Transition metals have high melting points.
Transition metals compounds are mostly coloured.
Sc3+ and Zn2+ are colourless in aqueous solutions.
Transition metals exhibit variable oxidation states.
Transition metal series exhibit fewer oxidation states at their extreme ends. (Sc, Ti, Cu)
Density of zinc is lower than that of copper.
Transition metals have high enthalpy of atomisation.
Generally speaking, the enthalpies of atomisation and melting points transition
metals of 3d, 4d and 5d series increase steadily down the group
Transition metals and their compounds show paramagnetic behaviour.
Hydrated copper sulphate is blue where as anhydrous copper sulphate is white.
The magnetic moments of the transition metals increase up to the middle of the series
and then decrease.
Transition metals generally form complex compounds.
Transition metals form interstitial compounds.
The third ionisation enthalpies of Mn & Zn are quite high in comparison to Fe.
Zn has little tendency to form complexes.
Zn, Cd and Hg are not considered as true transition metals.
[Ti(H2O)6]3+ is coloured while [Sc(H2O)6]3+ is colourless.
Mn2+ compounds are more stable than Fe2+ compounds towards oxidation to +3 state.
The decrease in atomic radius in the case of any d-series is not as large as that in
period 2 or 3.
A transition metal exhibits higher oxidation states in oxides and fluorides.
The d1 configuration is very unstable in ions of d-Block elements.
Scandium(II) is virtually unknown.
The magnetic moment of Co3+ is higher than that of Co2+.
The lowest oxide of transition metal is basic, the highest is acidic.
The densities of post-lanthanoid elements are very high.
The atomic sizes of corresponding elements of 4d and 5d series are almost the same.
La3+ and Lu3+ are colourless while rest of lanthanoids are coloured in the solid
as well as in aqueous state.
The first-ionisation enthalpies of the 5-d transition elements are higher than those of
the 4-d metals.
Cu+ is diamagnetic whereas Cu2+ is paramagnetic.
The ionisation enthalpy of Hf (6th period) is higher than that of Zr (5th period).
The most common oxidation state of lanthanoids is +3. However cerium and europium form additional oxidation states of +4 and +2 respectively.
Ce4+ is a good oxidant.
Eu2+ is a good reducing agent.
II. Describe the large scale preparation of:
1. Potassium dichromate from chromite ore (FeCr2O4).
2. KMnO4 manufactured from pyrolusite.
III. Write the chemical reactions involved.
1. Acidified potassium dichromate with (a) KI solution (b) FeSO4 solution.
2. Acidified potassium permanganate with (a) oxalate solution (b) Sn2+ solution.
3. Pr + H2O
4. Ce + N2 heated
5. Lu + O2
IV. Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
electronic configuration
oxidation state
atomic sizes
chemical reactivity.
V. What is lanthanoid contraction? How would you account for it?
What are its important consequences?
VI. Short Questions:
What are transition elements?
What is misch-metal?
Which is more stable – Mn2+ or Mn4+?
What are coinage metals?
Write the outer shell configuration of inner transition metals.
Calculate the magnetic moment of Mn2+.
Draw the structures of , Cr2O72- and CrO42-
Name the metal with the highest melting point.
Name the actinoid with no 5f electron.
Name the lanthanoid with the maximum paramagnetism.
What are the uses of lanthanoids?
What are transuranic elements?
INTERVIEW QUESTIONS YOU NEED TO CHOOSE
INTERVIEW SKILLS & HOW TO HANDLE DISCRIMINATORY QUESTIONS
QUESTIONS ARISING FROM THE REGULATORY RESPONSIBILITY BILL HON
Tags: questions, isomers, 1sketch, chemistry, coordination, structures
- INTERFERENCIAS LINGÜÍSTICAS DEL GALLEGO EN EL CASTELLANO EN UN
- LEARN TO USE THE BIBLE! THE PURPOSE OF THIS
- TITLE IMPACT DAMAGE DETECTION AND QUANTIFICATION IN CRFP LAMINATES
- MEDELLÍN OCTUBRE 26 DE 2020 SEÑORES MINCIENCIAS AV CALLE
- WARSZAWA 29082011 R ZESTAWIENIE OFERT ZŁOŻONYCH W KONKURSIE SZKOŁA
- FORMULARIO PARA DENUNCIA DE VIOLENCIA (LEY DE VIOLENCIA DE
- 22 AUGUST 2011 ROOKIE WEBB SCORES POINTS ON DEBUT
- FÖRETAG BESKED OM ANSTÄLLNINGS UPPHÖRANDE ELLER PERMITTERING (BYGGAVTALETS SAMT
- DISCURS D’INVESTIDURA DEL DIPUTAT H SR ARTUR MAS I
- RECORDS TRANSMITTAL AND RECEIPT COMPLETE AND SEND ORIGINAL
- Poder Legislativo ley n 253 que Aprueba el Convenio
- GOBIERNO DE CHILE SUBSECRETARIA DE TRANSPORTES PUBLICACIÓN Nº 160
- Procedimiento Para Obtener la Licencias de Edificación Procedimiento Para
- KALENDER PENDIDIKAN TAHUN PELAJARAN 20162017 MTS NEGERI BANTUL KOTA
- DECRETO 452018 DE 18 DE OCTUBRE POR EL QUE
- TYPICAL SPECIFICATION CLAUSE FOR 5000 SERIES RECESSED FLOOR ACCESSDUCTMULTIPART
- (IMIĘ I NAZWISKO) (MIEJSCOWOŚĆ) ………………………………………………………………(DATA) SĄD OKRĘGOWY W SUWAŁKACH
- QUESTIONS & ANSWERS OVER DE HEFFINGEN WIE MOET ZICH
- A LO LARGO DE TODO EL DÍA SE RECOGIERON
- NOTAS EXAMEN FINAL DERECHO CIVIL IV GRUPO B ALEMÁN
- (ESPACIO PARA EL LOGO DE LA EMPRESA INSTALADORA) BANAKO
- POSLANICA MLADIM OB 31 MAJU – SVETOVNEM DNEVU BREZ
- PYTANIA TESTOWE 20080615 231720 1 PROCESOR MA ARCHITEKTURĘ AKUMULATOROWĄ
- UNIVERSITY POLICIES AND PROCEDURES 080303 – RESTRICTED ANDOR PROHIBITED
- PRIVATE FEES FOR NONNHS WORK FROM APRIL 2018
- REPUBLIKA HRVATSKA KARLOVAČKA ŽUPANIJA OPĆINA CETINGRAD OPĆINSKO VIJEĆE S
- B&R ROZŠIŘUJE X20 IO SYSTÉM O MODULY S PROTOKOLEM
- HTTPBREMAIKFREEFR 2 A VIZ GOUERE SIZHUN 27 2007 BREMAIKFREEFR
- (IME I PREZIME) (ADRESA) TELMOB OIB
- ZWIĄZEK HARCERSTWA POLSKIEGO KATOWICE 26102006R KOMENDANT ŚLĄSKIEJ CHORĄGWI ROZKAZ
KEPUTUSAN PRESIDEN REPUBLIK INDONESIA NOMOR 7 TAHUN 1996 TENTANG
 U ANEXO 42 NIVERSIDADE DE ÉVORA MESTRADO EM ENSINO
U ANEXO 42 NIVERSIDADE DE ÉVORA MESTRADO EM ENSINO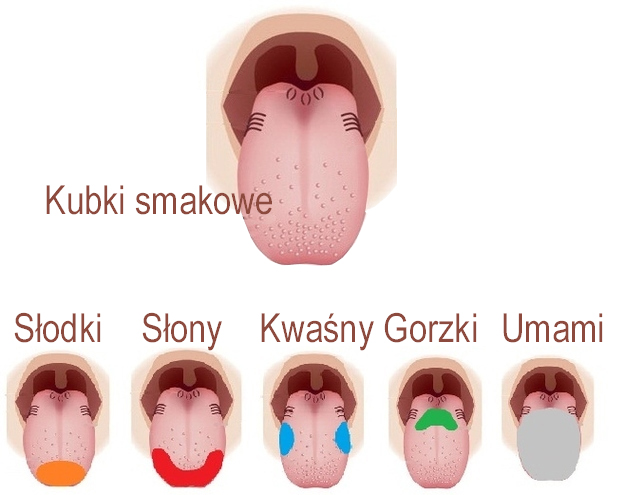 TEMAT OCENA JAKOŚCI POTRAW I NAPOJÓW OCENA ORGANOLEPTYCZNA 1
TEMAT OCENA JAKOŚCI POTRAW I NAPOJÓW OCENA ORGANOLEPTYCZNA 1VIRGINIA COMMONWEALTH UNIVERSITY THE L DOUGLAS WILDER SCHOOL OF
 WWF CONTACTED THE FOLLOWING COMPANIES TO SIGN ONTO WWF’S
WWF CONTACTED THE FOLLOWING COMPANIES TO SIGN ONTO WWF’S INSTALACIONES DE EQUIPOS A PRESIÓN CERTIFICADO DE INSPECCIÓN PERIODICA
INSTALACIONES DE EQUIPOS A PRESIÓN CERTIFICADO DE INSPECCIÓN PERIODICA ROAD MANAGEMENT PLAN ROAD INSPECTION CATEGORIES NOVEMBER 2009 ROAD
ROAD MANAGEMENT PLAN ROAD INSPECTION CATEGORIES NOVEMBER 2009 ROAD SOLICITUD DE APERTURA DE CRÉDITO DOCUMENTARIO IRREVOCABLE PÁG
SOLICITUD DE APERTURA DE CRÉDITO DOCUMENTARIO IRREVOCABLE PÁG  LA NORMA FISCAL DEFINE COMO RENDIMIENTOS DE TRABAJO PERSONAL
LA NORMA FISCAL DEFINE COMO RENDIMIENTOS DE TRABAJO PERSONAL VIII TÜRI VALLA KÜLADE TALVEMÄNGUD 2020 EESMÄRK 1 POPULARISEERIDA
VIII TÜRI VALLA KÜLADE TALVEMÄNGUD 2020 EESMÄRK 1 POPULARISEERIDA 58 DIRECCIÓN FIDUCIARIA SUBDIRECCIÓN DE OPERACIÓN TÉCNICA Y SEGUIMIENTO
58 DIRECCIÓN FIDUCIARIA SUBDIRECCIÓN DE OPERACIÓN TÉCNICA Y SEGUIMIENTOJUDY B GILBERT 19992000 SPEAK OUT! 25 PRONUNCIATION SIG
 TREAT PATIENTS IN A CLEAN AND SAFE ENVIRONMENT
TREAT PATIENTS IN A CLEAN AND SAFE ENVIRONMENT INFORME ALTERNATIVO AL CUARTO INFORME PERIÓDICO DE ARGENTINA ANTE
INFORME ALTERNATIVO AL CUARTO INFORME PERIÓDICO DE ARGENTINA ANTE MEMORIA DESCRIPTIVA 317LF013 CLIENTE ENOHSA ENTE NACIONAL DE OBRAS
MEMORIA DESCRIPTIVA 317LF013 CLIENTE ENOHSA ENTE NACIONAL DE OBRASSUPERINTENDENCIA DE BANCA Y SEGUROS REPORTE Nº 5 EMPRESA
REPUBLIKA HRVATSKA OSJEČKOBARANJSKA ŽUPANIJA OPĆINA KNEŽEVI VINOGRADI JEDINSTVENI
 RESOLUCIÓN CONASEV Nº 0552001EF9410 ANEXO I DE LAS
RESOLUCIÓN CONASEV Nº 0552001EF9410 ANEXO I DE LAS INSTITUTO CARLOS TEJEDOR ARTE ANÁLISIS Y PRODUCCIÓN DE LA
INSTITUTO CARLOS TEJEDOR ARTE ANÁLISIS Y PRODUCCIÓN DE LA LEY DE LA PROPIEDAD INDUSTRIAL CÁMARA DE DIPUTADOS DEL
LEY DE LA PROPIEDAD INDUSTRIAL CÁMARA DE DIPUTADOS DEL