2 OXIDES OXIDES CAN BE GROUPED ACCORDING TO
2 OXIDES OXIDES CAN BE GROUPED ACCORDING TODETERMINATION OF THE SOLUBILITY PRODUCT OF GROUPII HYDROXIDES INTRODUCTION
HAND SPECIMEN MINERALOGY LAB OXIDES FOR MINERALS IN
HIGH TEMPERATURE XRAY DIFFRACTION STUDY OF TANTALUM OXIDES PHASES
SYNTHETIC IRON OXIDES AN INDICATOR OF REDUCTION IN SOILS
Oxides
Oxides
Oxides can be grouped according to their chemical formulas, although members of each group may not necessarily be related through isomorphism or even genetically. Three main groups are recognized:
Oxides of the formula XO2
Rutile: A very common accessory mineral in igneous and metamorphic rocks. Is also common as a detrital mineral.
Cassiterite: (SnO2): A mineral that develops in pegmatites and high T hydrothermal deposits. Commonly associated with wolframite, molybdenite, and arsenopyrite in veins of quartz.
Pyrolusite (MnO2): Forms in the sedimentary environment by the oxidation of other Mn – bearing minerals. Common on the sea floor, in bogs, and on lake bottoms. Also found in veins with quartz.
Uraninite (UO2): Occurs in granites and pegmatites, as well as high T hydrothermal veins.
Oxides of the formula X2O3
All members of this group crystallize in the trigonal system, and are either tabular or prismatic. Hematite and ilmenite exhibit extensive (but incomplete) solid solution (Fig. 1).
Hematite: Widespread mineral in igneous and metamorphic rocks as well as hydrothermal veins. Also a common product of weathering, and chemical precipitation.
Ilmenite: Common accessory of igneous rocks. Common in some anorthosite massifs.
Corundum: As an accessory mineral in Al2O3 – rich, SiO2 - deficient igneous and metamorphic rocks. Rubies owe their colors to Cr3+, and therefore form in areas of regional or contact metamorphism of (or in the vicinity of) ultramafic rocks.
Spinel Group minerals XY2O4
Most members of the spinel group crystallize in the cubic system. They are characterized by extensive solid solution between their different end-members. Their compositions are therefore often represented on 3-D prisms (Fig. 2).
Magnetite (Fe3O4)- Ulvospinel (Fe2TiO4): Common accessory mineral in igneous and metamorphic rocks. Common constituent of banded iron formations.
Chromite (FeCr2O4): In mafic and ultramafic igneous rocks, often as pods, lenses or layers in lherzolites and harzburgites.
Spinel (MgAl2O4)– Hercynite (FeAl2O4): Common high T minerals in contact metamorphic rocks that are SiO2 – deficient.
Gahnite (ZnAl2O4)– Franklinite(ZnFe2O4): Gahnite like hercynite, is an accessory mineral in high T metamorphic rocks. Franklinite is restricted to the zinc deposits of Franklin, New Jersey.
Sulfides and Sulfosalts
Sulfosalts can be thought of as “double salts” in which As and Sb play the role of metals in the structure (i.e. they have a tendency to lose electrons). On the other hand, the same elements in most sulfides behave as non-metals; i.e. they have a tendency to gain electrons.
Examples: Arsenopyrite (FeAsS) is a sulfide. Its structure resembles that of marcasite (FeS2) in which one S has been replaced by As. On the other hand, Enargite (Cu3AsS4) is a sulfosalt in which As has lost 5 electrons. It can be thought of as a double salt: 3Cu2S.As2S5.
The system Cu - Fe - S:
Figure 3 is a plot of the compositions of the most important minerals in this system. The lines joining some of these minerals (known as tie lines) designate which minerals can coexist in equilibrium at specified conditions of P and T. The topology of these lines changes with changes in P - T conditions (Fig. 3b).
Troilite: (FeS) occurs in meteorites
Pyrrhotite: Fe1-xS: Most common as a disseminated sulfide in certain layers of large mafic and ultramafic rocks. Forms at relatively high T.
Pyrite: FeS2: Widespread mineral in igneous rocks and hydrothermal veins.
Chalcopyrite: CuFeS2: In high and medium T hydrothermal veins.
Bornite: Cu5FeS4: In high and medium T hydrothermal veins.
Chalcocite: Cu2S: A product of supergene enrichment of sulfides
Covellite: CuS: A product of supergene enrichment of sulfides.
Tags: oxides ======, ii. oxides, oxides, grouped, according
- ÇUKUROVA ÜNİVERSİTESİ SOSYAL BİLİMLER ENSTİTÜSÜ İŞLETME ANABİLİM DALI AİLE
- REGISTRO DE MIEMBROS 1 DATOS PERSONALES NOMBRE LUGAR DE
- MODELRELEASE FOTOKONTRAKT DENNE RELEASEKONTRAKT OMHANDLER FORHOLDENE VEDR FOTOGRAFERINGEN
- KVINOJA KVINOJA KI JO POZDRAVLJAJO KOT ČUDEŽNO ŽITO PRIHODNOSTI
- UPUTE O NAČINU PRAĆENJA I OCJENJIVANJA VJEROUČENIKA U OSNOVNOJ
- WHAT WILL BE THE GEOMETRY OF THE MAIN BENDS
- TEMPORARY METERED SUPPLY (TM) A TARIFF FOR TEMPORARY
- 1 DE 1 TÉRMINOS DE REFERENCIA PLAN INMEDIATO DE
- IMPLEMENTATION OF SHORTTERM FORECASTING MODELS IN THE NATURAL GAS
- ESTADÍSTICA I – PRÁCTICA 1 POWERPLUSWATERMARKOBJECT3 PRÁCTICA 2 ESTADÍSTICA
- ALLEGATO 2 MODELLO DI FORMULARIO PERIL DOCUMENTO DI
- NEGOCIACIÓN Y COMUNICACIÓN EN EL MERCADO INDIO NEGOCIACIÓN Y
- NORME DI PARTECIPAZIONE IL COMITATO PROVINCIALE DELLA LEGA CALCIO
- A HERO OF SCIENCE AFTER ALL MARCH 25 2010
- PUBLIC SPEAKING OBJECTIVES TO LEARN AND PRACTICE
- LAS PERSONAS CON DISCAPACIDAD HABLAN DE LA NUEVA GENÉTICA
- TEAM 1896 10 SAVING TIME AND MONEY SUMMARY WE
- SE HA INCREMENTADO EN UN 25 EL NÚMERO DE
- RECORDS DE ESPAÑA DE PESO MUERTO (MODALIDAD ÚNICA) 44
- “HE VENIDO A TRAER FUEGO A LA TIERRA…” (LC
- 0 1PIELIKUMS MINISTRU KABINETA 2009GADA NOTEIKUMIEM NR 981 INFORMĀCIJA
- ÖZEL GÜVENLİK HİZMETLERİNE DAİR KANUN KANUN NUMARASI 5188
- REVIEW OF THE TREATMENT OF OVERSEAS PENSIONS AND PAYMENT
- SISTEMAS DE INFORMACIÓN JUNIO 2010 IE 199509 NORMAS PARA
- UNIVERSIDAD DE BUENOS AIRES FACULTAD DE CIENCIAS SOCIALES SEMINARIO
- 12 AKNYSTOS SOCIALINĖS GLOBOS NAMAI PATVIRTINTA AKNYSTOS SOCIALINĖS GLOBOS
- 1819KO ESKOLARTEKO EUSKAL PILOTA TXAPELKETA JARDUNALDIA 17 EGUNA
- MAXIMAL SOFTWARE RELATED LINKS MAXIMAL SOFTWARE HOME SITE HTTPWWWMAXIMALSOFTWARECOM
- (TĖVŲ (GLOBĖJŲ) VARDAS PAVARDĖ) (ADRESAS TELEFONO NR)
- ANALISIS KEBUTUHAN ENERGI MOTOR LISTRIK PADA MOBIL HYBRID URBAN
 INSTITUTO SUPERIOR PARTICULAR INCORPORADO Nº 9110 “DE LA
INSTITUTO SUPERIOR PARTICULAR INCORPORADO Nº 9110 “DE LAPRAVILNIK O STATUSU STRANACA U REPUBLICI HRVATSKOJ (UREDNIČKI
 IOWA DEPARTMENT OF NATURAL RESOURCES LAND RECYCLING PROGRAM PARTICIPATION
IOWA DEPARTMENT OF NATURAL RESOURCES LAND RECYCLING PROGRAM PARTICIPATION S T P I INFORMATION TECHNOLOGY NEEDS ASSESSMENT FOR
S T P I INFORMATION TECHNOLOGY NEEDS ASSESSMENT FORGRANADA KONFERENCIA WELLNESS ÉS SPORTHOTEL A MAGYAR TRAUMATOLÓGUS TÁRSASÁG
 RECTANGLE 63 RECTANGLE 64 EIAVE APRIL 2017 TABLE OF
RECTANGLE 63 RECTANGLE 64 EIAVE APRIL 2017 TABLE OFLIST OF ADDITIONAL NATIONAL REQUIREMENTS – ON TOP OF
 CONSTITUCION DE EMPRESAS POR VIA TELEMATICA (SLNE Y SRL)
CONSTITUCION DE EMPRESAS POR VIA TELEMATICA (SLNE Y SRL)LECTURE 12 SOME COMMON COMPLICATIONS IN DENTAL CLINICAL PRACTICE
WYMAGANIA EDUKACYJNE Z BIOLOGII DLA KLASY 5 SZKOŁY PODSTAWOWEJ
 EVOLUCIÓN HACIA LA NUEVA INTERNET A PRINCIPIOS DE LOS
EVOLUCIÓN HACIA LA NUEVA INTERNET A PRINCIPIOS DE LOSDOUG SMITH BIO DOUG SMITH HAS BEEN INVOLVED IN
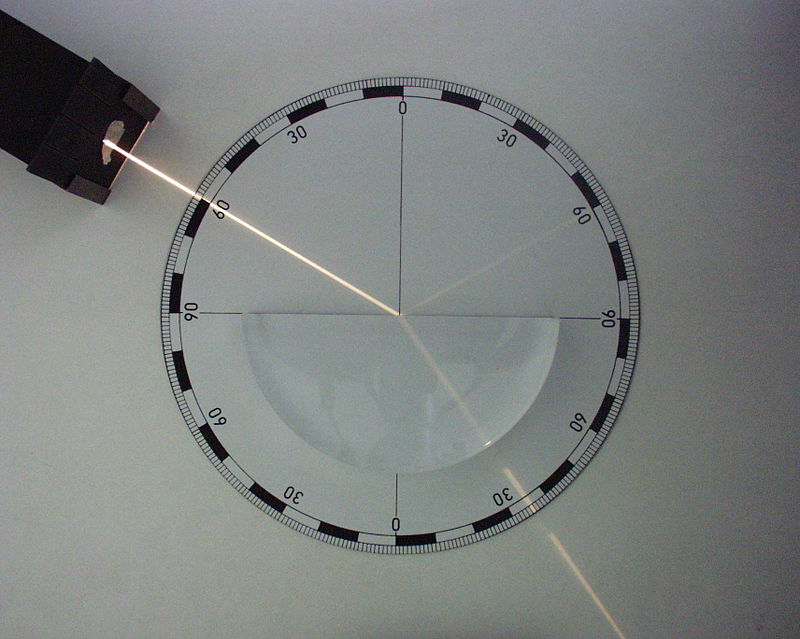 FÉNYVISSZAVERŐDÉS A FÉNYVISSZAVERŐDÉS (REFLEXIÓ) EGY OPTIKAI JELENSÉG HA A
FÉNYVISSZAVERŐDÉS A FÉNYVISSZAVERŐDÉS (REFLEXIÓ) EGY OPTIKAI JELENSÉG HA A SCHULINTERNER LEHRPLAN ZUM KERNLEHRPLAN FÜR DIE SEKUNDARSTUFE I (G9)
SCHULINTERNER LEHRPLAN ZUM KERNLEHRPLAN FÜR DIE SEKUNDARSTUFE I (G9)PROCESO DE EXPERIMENTACIÓN O ACTIVIDAD PELIGROSA TRASVASE DE FORMALDEHÍDO
DOWNLOAD URL IS NOT CORRECT
 HB 1823 STRICKEN LANGUAGE WOULD BE DELETED FROM PRESENT
HB 1823 STRICKEN LANGUAGE WOULD BE DELETED FROM PRESENT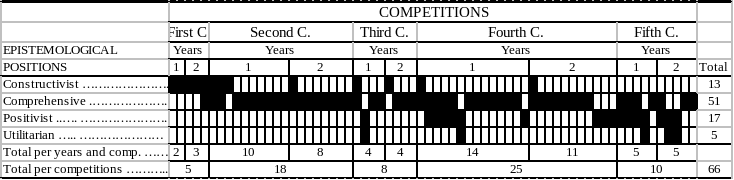 EPISTEMOLOGICAL PLURALISM EPISTEMOLOGICAL PLURALISM AND FAIRNESS IN PEER EVALUATION
EPISTEMOLOGICAL PLURALISM EPISTEMOLOGICAL PLURALISM AND FAIRNESS IN PEER EVALUATION1 E ANEXO N° 4 INSTRUCCIONES FORMULARIO 1806 DECLARACIÓN
(ANTETUL UNITĂŢII CARE ÎŞI EXPRIMĂ INTENŢIA) NR ÎNREGISTRARE