PRACTICE GUIDELINE INFORMATION SHEET GUIDELINE TITLE KAISER PERMANENTE
GUIDES OF GOOD PRACTICE ORGANISING COMMONERS ASSOCIATIONSINTRODUCTION THIS PRACTICE GUIDE OUTLINES VARIOUS CONSULTATIVE
PRACTICE NOTE SOURCING SUPPLEMENTARY EMERGENCY RESPONSE RESOURCES
& COUNCIL OF CIVIL SERVICE UNIONS GOOD PRACTICE FOR
(4021225) 8 N1260(E)(J14)H NATIONAL CERTIFICATE OFFICE PRACTICE N5 (4021225)
(still) More Naming Practice Write the Names of the
Practice Guideline Information Sheet
Practice Guideline Information Sheet
Guideline Title
|
Kaiser Permanente Colorado Region Meningococcal Vaccine Guideline
|
Date Submitted Approved By: (Dept & Person)
|
July, 2003 |
|
The Prevention Committee; Eric France, MD, MSPH |
Contact person to contact regarding this guideline’s content
|
Nancy Larson, RN, MS |
||||
Department |
The Prevention Department |
|||
Phone |
303-344-7382 |
|
||
Author(s)
|
Eric France, MD & Nancy Larson, RN, MS |
Clinical Category Choose one (more if needed) from the list below.
|
Allergy |
Geriatrics |
Pharmacy |
|
Anesthesiology |
Hematology/Oncology |
Physiatry/Rehab |
|
Cardiology |
Infectious Disease |
Podiatry |
|
Cardiovascular Surgery |
Mental Health |
Pulmonology |
|
Dermatology |
Nephrology |
Radiation Oncology |
|
ENT/Head and Neck Surgery |
Neurology |
Radiology |
|
Emergency Care |
Neurosurgery |
Rheumatology |
|
Endocrinology |
Obstetrics/Gynecology |
Surgery |
|
Eye Care |
Orthopedics |
Urology |
|
Gastroenterology |
Pathology/Laboratory Services |
Primary Care |
Keywords To aid in searching for the guideline
|
meningococcal, vaccine, meningitis |
Abstract or other summary description of this guideline
|
Students entering college, especially those who will be living in dormitories, and their parents should be informed during routine prematriculation medical visits about the increased risk of meningococcal disease and potential benefits of immunization as well as limitations of the vaccine, primarily the lack of protection against serotype B meningococcal disease. Students who wish to be immunized should be vaccinated.
|
Intended Audience
|
All Providers |
Physicians |
|
All Providers and Staff |
Nurses |
|
Members |
Physician Assistants |
|
Other: |
|
Practice Guideline Plan Sheet
What is the intended clinical outcome of implementing this guideline?
|
That all students entering college and planning to live in a dormitory situation have been appraised of their slightly increased risk of developing meningococcal disease and the availability and limitations of a vaccine to prevent some strains of meningococcal disease. |
What is the implementation plan?
|
The guideline was initially implemented in April 2001.
The guideline is accessible on the PKC Connection and the Nursing Website. Many colleges highly suggest or require the vaccine for admission of newly enrolled students. . |
What is the plan for evaluating the outcome of implementing this guideline?
|
Periodic chart audits.
The Immunization Task Force and The Pharmacy Department centralized distribution of the vaccine to medical clinic pharmacies, which allows close monitoring of usage. Approximately 3,200 doses are given per year.
|
Practice Guideline Attachment Sheet
Attachment One: Reference Document
-
Kaiser Permanente Colorado Region
Meningococcal Vaccine Guideline
Adoption Date: 4/01 Page #1
Responsible Parties: Eric France MD MSPH, Chair, Immunization Task Force
Nancy Larson RN, Co-Chair Immunization Task Force
Review Date: 7/03 Next review Date:7/05
These guidelines are informational only and are not intended or designed to substitute the reasonable exercise of independent clinical judgment by providers in any particular set of circumstances for each patient encounter. The guidelines are flexible and are intended to be used as a resource for integration with a sound exercise of clinical judgment. They can be used to create an approach to care that is unique to the needs of each individual patient.
Rationale for Guideline: Studies conducted in college age students have shown that there is an elevated risk for sporadic meningococcal disease in this population. Freshmen living in dormitories have an increased incidence of meningococcal disease of 4.6 per 100,000 compared to a rate of 1 per 100,000 for similar aged young adults. A safe and effective quadrivalent polysaccharide vaccine exists that is ≥ 85% effective against the covered serotypes.
Target Population: College age students and people considered high-risk.
Recommendation: Specific recommendations for use of the meningococcal vaccine are as follows:
Students entering college, especially those who will be living in dormitories, and their parents should be informed during routine prematriculation medical visits about the increased risk of meningococcal disease and potential benefits of immunization as well as limitations of the vaccine, primarily the lack of protection against serotype B meningococcal disease.
Students who wish to be immunized should be vaccinated.
Immunization is not recommended for students who will not be living in dormitories because their risk of meningococcal disease is not increased relative to that of persons of similar age in the general population. However, immunization is not contra-indicated for these students and vaccine may be given if requested.
Routine reimmunization of college students who were immunized as freshmen is not indicated. However, for those who were immunized 3 to 5 years previously and are or will be in high-risk circumstances, such as travel to geographic areas with hyperendemic or epidemic meningococcal disease, reimmunization should be considered. Similarly, vaccine should be considered for matriculating freshmen who were immunized 3 or more years previously and will be living in dormitories.
People for whom the vaccine is recommended to be routinely given includes those populations considered high risk. High risk patients include people with underlying immune deficiencies, including HIV, terminal complement component deficiencies, anatomic or functional asplenia, and patients traveling to countries where the disease is considered common or endemic. Persons working in research, industrial or clinical laboratories that are routinely exposed to Neisseria meningitidis that may be aerosolized should consider vaccination.
Since the vaccine is a polysaccharide vaccine, it is not recommended for use in children < 2 years of age. The vaccine is relatively ineffective and has a relatively short duration of protection in this age group.
The vaccine is recommended to control serogroup C meningococcal outbreaks. An outbreak is defined as 3 or more confirmed or probable cases of serogroup C disease in a period of 3 months, with a resulting primary attack rate of at least 10 cases per 100,000 persons.
Performance measurement: The number of meningococcal vaccines administered by the Kaiser Permanente Colorado Region this year will be compared to the number of meningococcal vaccines administered the previous year.
References
CDC. Prevention and Control of Meningococcal Disease and Meningococcal Disease and College Students: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2000;49(No. RR-7): 1-20.
American Academy of Pediatrics. Meningococcal disease prevention and control strategies for practice-based physicians. Pediatrics. 2000;106:1500-1504.
From
CDC. MMWR 2000;49(No.RR-7).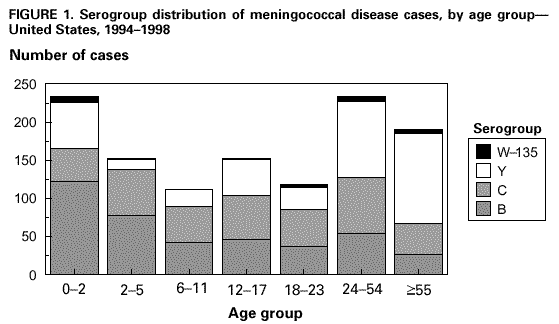

X Additional Attachments:
X Patient education materials: available in Standard Stock:
Kaiser Permanente Patient Education Sheet SS# 00233497.
CDC VIS Sheet SS# 00220018.
1 VAT PRACTICES WITHIN SACU AND POSSIBILITIES FOR
1 APPROPRIATE USE OF RESTRICTIVE PRACTICES AND WITHDRAWAL SPACES
1 INTERNATIONAL AIR TRANSPORTATION FAIR COMPETITIVE PRACTICES ACT THE
Tags: guideline information, vaccine guideline, guideline, sheet, title, kaiser, permanente, information, practice
- Lievegoed Formas Sociales con el Ejemplo de Instituciones de
- 20200519cuadroparaaportacionesprdvfu_tcm30-509343
- FORMULIR 3 KOP INSTANSI SURAT IZIN ATASAN YANG BERTANDA
- NAME MODERN MARVELS GEORGE WASHINGTON CARVER TECH 1
- ACTIVIDADES ADICAE COMUNIDAD VALENCIANA LUNES 29 DE SEPTIEMBRE 1
- DOF 110497 ACUERDO QUE ESTABLECE LA INFORMACIÓN RELATIVA A
- COORDINATION SUD COORDINATION SUDS POSITION ON THE
- ELI LILLY ANNOUNCES HUMULIN® N AND 7030 PENS PHASE
- BİRLEŞME BIRLEŞME DEVROLUNAN ŞIRKETIN MALVARLIĞI KARŞILIĞINDA BIR DEĞIŞIM ORANINA
- PATVIRTINTA ŽEMAITIJOS NACIONALINIO PARKO DIREKTORIAUS 2016 M SPALIO 3
- EXSC 8124 ANSI EXSC INTERPRETATION DISCUSSION OF BALANCE WITHIN
- FOTO 3 X 4 HOJA DE MATRICULA AÑO LECTIVO
- “LIKELY” IN THE HARPER SECTION 46 HON PETER HEEREY
- PROSEDUR MUTU JUDUL PROSEDUR MAHASISWA PINDAH DARI PT
- VARTOTOJŲ IR FINANSŲ RINKOS DALYVIŲ GINČŲ NETEISMINIO SPRENDIMO PROCEDŪROS
- ZAŁĄCZNIK NR 1 DO UMOWY …082021 ZAŁĄCZNIK NR 1
- STEPPING STONE SENIOR CENTRE DISCIPLINES POLICY INFRACTIONS AND SANCTIONS
- PLAN DE PARTO Y NACIMIENTO EN LA CLÍNICA IMQ
- VALIDAÇÃO DE ARQUIVOS HTML UM DOCUMENTO HTML VÁLIDO DECLARA
- LA ADOLESCENCIA ES UNA DE LAS ETAPAS MÁS COMPLEJAS
- DZIEŃ PAMIĘCI OFIAR ZBRODNI KATYŃSKIEJ 13 KWIETNIA OBCHODZILIŚMY DZIEŃ
- Poetry Rubric Writing and Illustrating a Poem Name Beginning
- 8º ANO EF TERESA 040518 ESPANHOL LOS SUPERLATIVOS
- ATA DA 43ª REUNIÃO ORDINÁRIA DE 23 DE NOVEMBRO
- INVENTARIO DE RIESGOS CRITICOS RGGE01300 ACTIVIDADES PELIGRO RIESGOS
- TEMELJEM ČLANKA 25 ALINEJA 7 PRAVILNIKA NOGOMETNOG SAVEZA VELIKE
- RUSLAND OPDATERINGSDATO 17122014 NATURERHVERVSTYRELSEN HAR SKÆRPET FOKUS PÅ ALLE
- MAILING LABEL FIRST CLASS MAIL TO IPI FARMS OFFICE
- BODI FIT ODMOR TOREK – MOČ NOG NA STEŽAJ
- MŰSZAKI MELLÉKLET 2051 BIATORBÁGY YBL MIKLÓS SÉTÁNY HRSZ129785 AZ
 MUUTOSILMOITUS SAAPUNUT MUUTOSILMOITUS – OPETUSTUNTIEN SIIRTYMINEN TAI PERUUNTUMINEN
MUUTOSILMOITUS SAAPUNUT MUUTOSILMOITUS – OPETUSTUNTIEN SIIRTYMINEN TAI PERUUNTUMINENİŞ MAKİNASI TESCİL TALEP FORMU (YALOVA TICARET VE
BULLETIN NR 072 V 22 JAN 2004 BPRÄS
NUEVOS MODELOS ASOCIATIVOS PARA NUEVOS TIEMPOS YA VIENE SIENDO
BOSTON TOASTMASTERS LEADERSHIP INSTITUTE FEBRUARY 19 2003 RUTH LEVITSKY
PROXECTO DE DIRECCIÓN IES AUGA DA LAXE DE GONDOMAR
 MACRO PROCESO ADMINISTRATIVO PROCESO GESTION DE BIBLIOTECAS PROCEDIMIENTO PROCESOS
MACRO PROCESO ADMINISTRATIVO PROCESO GESTION DE BIBLIOTECAS PROCEDIMIENTO PROCESOSČJ SO22911601 PŘÍLOHA PŘÍKAZNÍ SMLOUVA Č SMLOUVY UZAVŘENÁ
INFORMACIJA DĖL MINIMALIŲ KVALIFIKACINIŲ VALSTYBINIŲ MOKSLO IR STUDIJŲ INSTITUCIJŲ
LA GACETA 136 –MIÉRCOLES 15 DE JULIO 2009 DECRETO
 YÜKSEKÖĞRETİM KURULU BAŞKANLIĞI YÜKSEKÖĞRETİM KURUMLARI FAALİYET RAPORU HAZIRLAMA REHBERİ
YÜKSEKÖĞRETİM KURULU BAŞKANLIĞI YÜKSEKÖĞRETİM KURUMLARI FAALİYET RAPORU HAZIRLAMA REHBERİJOHN 316 MISSION STAFF POSITION & DESCRIPTION POSITION KITCHEN
WYKAZ NOCLEGOWNI SCHRONISK I JADŁODAJNI NA TERENIE KALISZA
THE DUTY TO CARE FOR HISTORIC BUILDINGS CHARLES
MODELO ACTA DE ASEMBLEA CONSTITUINTE EN ÁS
26 UNIVERSITÉ DE TOULOUSE II LE MIRAIL DÉPARTEMENT
PATVIRTINTA VIEŠOSIOS ĮSTAIGOS DRUSKININKŲ TURIZMO IR VERSLO INFORMACIJOS CENTRO
ASSESSMENT OF PERIODIC SAFETY UPDATE REPORTS FOR NATIONALLY AUTHORISED
 WHAT PARAMETERS INCREASE OR DECREASE POWER? THIS DEMO ILLUSTRATES
WHAT PARAMETERS INCREASE OR DECREASE POWER? THIS DEMO ILLUSTRATES1 UNIVERSIDAD DE BUENOS AIRES 2 FACULTAD DE FILOSOFÍA