PROCEDURE 523 MULTISITE APPROVAL RESEARCH GOVERNANCE UNIT ST VINCENT’S
ALL SCHOOL PROCEDURES AND POLICIES FROM THE STUDENTDISCHARGE OF CARE ORDERS – STANDARD PROCEDURE THE
ILLICIT DISCHARGE DETECTION AND ELIMINATION FIELD PROCEDURES AND
OFFICE OF STUDENT EMPLOYMENT PROCEDURE FOR GRADUATE
Procedures for Varying Shared Ownership Leases Background
YOUTH OFFENDING SERVICE HOME VISITLONE WORKING PROCEDURE
Patient Admission
Procedure 5.23
MULTISITE APPROVAL
|
|
RESEARCH GOVERNANCE UNIT St. Vincent’s Hospital (Melbourne) Caritas Christi Hospice St. George’s Health Service Prague House |
MULTISITE APPROVAL
Statement of Intent and Outcomes
The St Vincent’s Hospital Human Research Ethics Committee is committed to fulfilling Section 5 of The National Statement on Ethical Conduct in Human Research (2007, updated 2018) by reducing the duplication of ethical review where possible.
Definitions
ERM is defined as the Ethical Review Manager, is an information management system
for all ethics and research governance, throughout the life of a research
project.
Reviewing HREC is defined as the HREC who is allocated to undertake an ethical review of the study.
Accepting HREC is defined as other HRECs who accept the ethical approval of the reviewing HREC without further ethical review.
Lead Site is defined as the single site responsible for the ethical submission of documents for a study.
Master Participant Information and Consent Form is defined at the universal template which must be used by all sites participating in the study. This document must not contain site specific information.
Site Specific Participant Information and Consent Form is the universal template which must be used by all sites participating in the study, which has been modified to include site specific information (i.e. institution name, investigators name, contact details)
Procedure
The Streamlined Ethical Review Process for multisite clinical trials is a dual initiative of the Victorian, New South Wales, Queensland and South Australian Governments. This allows clinical trials to be reviewed by one HREC with the decision mutually accepted by other certified centres. This ensures that ethical review is sought once only and that each participating site need only obtain governance authorisation prior to the commencement of the study via the submission of a Site Specific Assessment (SSA).
St Vincent’s Hospital is committed to this multisite approval process, and is certified to both review and accept the approval of other centres for multicentre trials. At all times, participating sites must adhere to the Standard Operating Procedures which are available from https://www2.health.vic.gov.au/about/clinical-trials-and-research/clinical-trial-research
The accredited HRECs which participate in the multisite approval pathway are listed in the Memorandum of Understanding, which can be accessed from the Victorian Department of Health website https://www2.health.vic.gov.au/about/clinical-trials-and-research/clinical-trial-research
HREC Review
The lead site is responsible for submitting the ethics application to the reviewing HREC. This is achieved by contacting the Office of Health and Medical Research (OHMR), who is responsible for registering all studies for consideration and allocating them to a HREC for ethical review. This ensures an unbiased even distribution of work load.
Once the study is allocated to a reviewing HREC, the lead site must submit the required documents for review.
Documents for St Vincent’s Hospital (Melbourne) to provide ethical review can be found from this link: https://www.svhm.org.au/research/researchers/human-research-ethics-committee
Once a valid submission is received by the reviewing HREC, the Principal Investigator will be formally notified in writing to confirm the validity of the study and to confirm the meeting date at which the study will be reviewed. ERM will also be updated to ensure the study details are entered, to confirm the validity of the study, and to allocate the study to a meeting. The clock must commence running at this stage.
A hard copy study file is then devised. All SERP study files must be marked with an “S” for the purposes of identification, and contain a sticker on the front of the file denoting whether the HREC is the “reviewing HREC”, or “accepting HREC”. This must also be recorded within the Research Governance Unit database.
Once ethical review has occurred, the Principal Investigator will be formally notified of the outcome in writing. ERM must also be updated with the outcome, and the clock stopped.
Once the study has been ethically approved, a formal letter of approval must be sent to the Principal Investigator, along with a copy of the approved documents. This must be sent electronically, to allow the Principal Investigator to circulate the documents to the Sponsor (as applicable) Investigators at other participating sites, and the Research Governance Offices at each site where the study will be conducted.
Once approval is granted, other sites should commence the submission of SSA applications to other participating sites, using the approved documents as templates.
SSA Review
Once ethical approval has been obtained from an accredited HREC, a Site Specific Assessment application must be submitted by each participating site to their respective Research Governance Office, for governance review only.
SSA Applications must include the letter of approval from the lead site, all ethically approved documents, a site specific PICF and any legal documents including Clinical Trial Agreements, Insurance Certificates and CTN forms.
Once governance authorization has been granted, the site Principal Investigator will be notified in writing. ERM must also be updated to record the authorization.
Amendments
Where St Vincent’s Hospital is the reviewing HREC, a full submission of the amendment is required for review and formal ethical approval. Once the amendment has been approved, a formal letter of approval must be sent to the Principal Investigator, along with a copy of the approved documents. This must be sent electronically. Approval must also be recorded in ERM.
Where St Vincent’s Hospital is the accepting HREC, a full submission of the amendment along with the HREC approval letter is required for governance review. Once the amendment has been approved, a formal letter of approval must be sent to the site Principal Investigator.
Adverse Events
Where St Vincent’s Hospital is the reviewing HREC, a full submission of any related, potentially related or possibly related Suspected Unexpected Serious Adverse Reaction (SUSAR) as well as line listings and annual safety reports are required for review and formal ethical approval.
Once the adverse event has been approved, a formal letter must be sent to the Principal Investigator. This must be sent electronically.
All adverse events occurring at Vincent’s Hospital must be reported to the HREC as soon as possible.
These events must be reported to the Victorian Managed Insurance Authority (VMIA) by the Research Governance Unit to comply with local insurance requirements.
Where St Vincent’s Hospital is the accepting HREC, only those adverse events occurring at Vincent’s Hospital or those that have an impact on the conduct of the trial must be reported as soon as possible.
Associated Procedures/Instructions
Procedure 5.13 – Monitoring Approved Research
Procedure 5.7 – Document and Record Management
Procedure 5.11 – Minimising Duplication of Ethical Review
Reference Documents
The National Statement on Ethical Conduct in Research Involving Humans in accordance with the NHMRC Act, 2007 (Cth)
Australian Code for the Responsible Conduct of Research (2018)
Authorized by:
Dr Megan Robertson
Director of Research
|
Author: Dr Tam Nguyen, Executive Officer |
|
|
Date Issued: 2011 |
Next Review: 2020 |
|
Date Revised: 2018 |
Filepath: |
5.23
Multisite Approvals, 2018 Page
(REPORT TEMPLATE) INDEPENDENT ACCOUNTANT’S REPORT ON APPLYING AGREEDUPON PROCEDURES
(YOUR BUSINESS NAME HERE) – SAFE WORK PROCEDURE
(YOUR BUSINESS NAME HERE) – SAFE WORK PROCEDURE PORTABLE
Tags: approval research, of approval, governance, vincent’s, procedure, research, approval, multisite
- 8 TERAPIA DE ACEPTACIÓN Y COMPROMISO PARA VERGÜENZA Y
- PERATURAN BUPATI KUNINGAN NOMOR 44 TAHUN 2013 TENTANG
- O BČINA DOBJE OBČINA DOBJE DOBJE PRI PLANINI
- NAČRT IZOBRAŽEVANJA DELAVCEV ŠOLE V ŠOLSKEM LETU 20102011
- DATE MR WILLIAM SMITH OAK CITY COMMUNITY FOUNDATION 111
- PRAVILNIK O OBJAVAH POGODB S PODROČJA JAVNEGA NAROČANJA IN
- CUADERNILLO DE TRABAJO LENGUAJE 2º BÁSICO N°4 NOMBRE
- CUMBERLAND HOUSE SURGERY DID NOT ATTEND POLICY INTRODUCTION
- ZAŁĄCZNIK NR 9 DO SIWZ KARTA PRZEKAZANIA ODPADÓW KARTA
- OFSTED AGORA 6 CUMBERLAND PLACE NOTTINGHAM NG1 6HJ T
- WYNIKI VII EDYCJI KONKURSU PRZYRODNICZOARTYSTYCZNEGO „PRZYRODNICZE RYMOWANIE OTACZAJĄCEGO
- RECURSOS HUMANOS SERVICIO DE SALUD VIÑA DEL MARQUILLOTA HOSPITAL
- ZAŁĄCZNIK DO INFORMACJI DLA ZARZĄDU OGŁOSZENIE W SPRAWIE SKŁADANIA
- GENERALITAT DE CATALUNYA RN B0178B0206 DEPARTAMENT DE BENESTAR SOCIAL
- CUESTIONARIO ( CUALIDADES DEL DELEGADOA) LA ELECCIÓN DE DELEGADO
- 41 FOX CURRICULUM VITAE REBECCA KANAK FOX PH D
- NONPROFIT AND VOLUNTARY SECTOR QUARTERLY VOLUME 50 ISSUE 3
- LINEAMIENTOS AL MARGEN UN SELLO QUE DICE INSTITUTO DE
- CÁTEDRA LATINOAMERICANA COMUNICACIÓN DEMOCRACIA CIUDADANÍA Y TECNICA DÍAS 1
- PRUEBA PUNTUABLE PARA EL CTO DE ESPAÑA DE POWERLIFTING
- EXCMO AYTO DE CUENCA REF SECRETARÍA GENERAL NOTAEXTRACTO DE
- POLÍTICA DE COOKIES Y ALMACENAMIENTO LOCAL NAVEGAR POR
- CUMBERLAND GOODWILL EMS 519 SOUTH HANOVER STREET CARLISLE PA
- I NZQA APPROVED NTERNAL ASSESSMENT RESOURCE MAKING MUSIC 24B
- RAYMOND CARVER (19391988) MIEDO MIEDO DE VER UNA PATRULLA
- THE FOLLOWING ANNEXES SHOULD BE READ IN CONJUNCTION WITH
- PAUL F SAVICH TRUSTEE ERNEST HAEFELE BALM BOYETTE ROAD
- EAST HANOVER SOCCER CLUB LEADERSHIP AND COMMITMENT COACHES’ CODE
- TÁJÉKOZTATÓ AZ AUTONÓM VÁMFELFÜGGESZTÉSEKRŐL ÉS VÁMKONTINGENSEKRŐL A KÖZÖSSÉGI FELDOLGOZÓIPAR
- NEBRASKA ENVIRONMENTAL TRUST REVISED MARCH 2016 GENERAL GRANT APPLICATION
 PATVIRTINTA TRAKŲ R LENTVARIO PRADINĖS MOKYKLOS DIREKTORIAUS 2017 M
PATVIRTINTA TRAKŲ R LENTVARIO PRADINĖS MOKYKLOS DIREKTORIAUS 2017 M LYCÉE RABELAIS MEUDON ELECTIONS DES DÉLÉGUÉS DE CLASSES
LYCÉE RABELAIS MEUDON ELECTIONS DES DÉLÉGUÉS DE CLASSES23 WORLDS OF PAPER AN INTRODUCTION ISABELLE CHARMANTIER THE
 BIEDRĪBAS LUDZAS RAJONA PARTNERĪBA PAZIŅOJUMS BIEDRĪBA LUDZAS RAJONA PARTNERĪBA
BIEDRĪBAS LUDZAS RAJONA PARTNERĪBA PAZIŅOJUMS BIEDRĪBA LUDZAS RAJONA PARTNERĪBA PARLAMENTO EUROPEO 2009 2014 DOCUMENTO DE SESIÓN DATE{08022012}822012DATE
PARLAMENTO EUROPEO 2009 2014 DOCUMENTO DE SESIÓN DATE{08022012}822012DATE13 EDITOR’S NOTE ROBBIE PAUL GAVE UI CALS PERMISSION
EXAMEN DE ORTOGRAFÍA 2017 LEE CON CUIDADO Y MARCA
 CONSELLERÍA DE EDUCACIÓN E ORDENACIÓN UNIVERSITARIA IES NOME DO
CONSELLERÍA DE EDUCACIÓN E ORDENACIÓN UNIVERSITARIA IES NOME DOCURRICULUM VITAE INFORMACIÓN PERSONAL APELLIDO Y NOMBRES PANARO
RELAXATION TECHNIQUES THE WORLD IS STRESSFUL BUT WE
 MUNICIPALIDAD DE ROSARIO SECRETARÍA DE OBRAS PÚBLICAS MUNICIPALIDAD DE
MUNICIPALIDAD DE ROSARIO SECRETARÍA DE OBRAS PÚBLICAS MUNICIPALIDAD DE SECRETARY’S REPORT NO 2 MAY 9 2007 REPORT OF
SECRETARY’S REPORT NO 2 MAY 9 2007 REPORT OF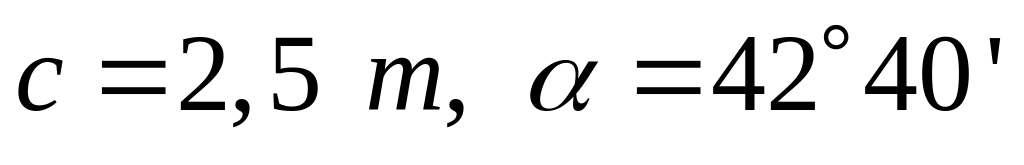 PRIPRAVA NA PISNO OCENJEVANJE ZNANJA IZ MATEMATIKE SKLOP METRIČNA
PRIPRAVA NA PISNO OCENJEVANJE ZNANJA IZ MATEMATIKE SKLOP METRIČNAINSS651 CH 12 DISTRIBUTED DATABASES DDB A DB PHYSICALLY
 OPRACOWANIE MGR AGNIESZKA LIPIEC XXIII LICEUM OGÓLNOKSZTAŁCĄCE IM NAUCZYCIELI
OPRACOWANIE MGR AGNIESZKA LIPIEC XXIII LICEUM OGÓLNOKSZTAŁCĄCE IM NAUCZYCIELI22360 VERSION 1 PAGE 3 OF 3 RIDE A
UMA POÉTICA DA VOZ CONCEITOS PARA ESTUDO DA LÍRICA
RUHR – UNIVERSITÄT BOCHUM INSTITUT FÜR POLITIKWISSENSCHAFT LEKTÜREKURS FEMINISTISCHE
 7º PROGRAMA MARCO DE I+D ALIMENTACIÓN AGRICULTURA Y PESCA
7º PROGRAMA MARCO DE I+D ALIMENTACIÓN AGRICULTURA Y PESCA TRAINING & POLICY MANUAL FOR SMALL ANIMAL VOLUNTEERS OBJECTIVE
TRAINING & POLICY MANUAL FOR SMALL ANIMAL VOLUNTEERS OBJECTIVE
