PHYSICAL PROPERTIES – MELTING AND BOILING POINT ESSENTIAL STANDARD
OVERWEIGHT OBESITY AND LACK OF PHYSICAL ACTIVITYFACULTY OF ENGINEERING AND PHYSICAL SCIENCES TAUGHT
INTER INTERNATIONAL FEDERATION OF ADAPTED PHYSICAL ACTIVITY
PHYSICAL THERAPY REFERRAL FORM SECONDARY STUDENT’S NAME
1 CWT PROGRAM LOCATION SPECIFIC CITY COLUMBIA 2 PHYSICAL
1 EFFECTS OF TAPERING ON PHYSICAL MATCH PERFORMANCE IN
Section 2
Physical Properties – Melting and Boiling Point
Essential Standard Understand: Phase Change, Physical Properties of Matter and Physical and Chemical Properties.
Clarifying Objective: Compare the physical properties of pure substances that are independent of the amount of matter present including density, melting point, boiling point and solubility to properties that are dependent on the amount of matter present to include volume, mass and weight.
Learning Goal: Understand that different substances have unique physical properties.
Mini-Lesson #1:
State whether each of the following changes is a “chemical change” or “physical change”.
Breaking a pencil lead
Crumpling a piece of paper
Burning a match
A nail rusting
Digesting your food
Active Learning (whole class and independent):
Complete Melting and Boiling Point Investigation by color-coding the thermometer graphs as directed.
Reflection (independent):
Answer the questions on the back of the melting and boiling point sheet.
Fill in the “Own Words” definitions of melting point and boiling point on your vocabulary list.
Name________________________________________ CP _____ Date_______
Melting and Boiling Point Investigation
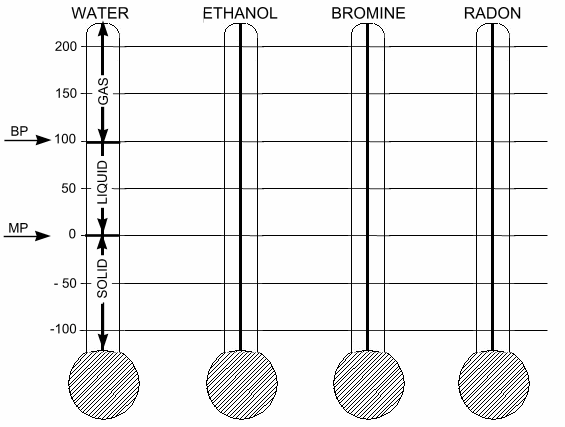
Color the thermometer graphs according to the instructions at the bottom of this page. Some of the information has already been put onto the “Water” graph for you. Then, answer the questions on the back of this page.
|
Substance |
Melting
Point |
Boiling
Point |
|
Water |
0 |
100 |
|
Ethanol |
–115 |
78 |
|
Bromine |
–7 |
59 |
|
Radon |
–71 |
–61 |
For each substance:
Use a pencil to darken the melting point and boiling point lines.
With a yellow pencil, color in the area of the thermometer directly above the boiling point line.
With a red pencil, color in the area of the thermometer between the boiling point and melting point lines.
With a blue pencil, color in the area of the thermometer below the melting point line.
Use a pencil to add arrows to indicate the melting and boiling points. Place the arrows to the left of each thermometer and label them "BP" and "MP."
In each of the yellow regions, write the word gas.
In each of the red regions, write the word liquid.
In each of the blue regions, write the word solid.
True or False
True |
False |
|
|
Bromine is a gas at – 60 °C. |
||
|
Radon is a solid at – 100 °C. |
||
|
Ethanol is a gas at 140 °C. |
||
|
Water is a liquid at – 5 °C. |
||
|
Bromine is a solid at 0 °C. |
||
|
Radon melts at a lower temperature than water. |
||
|
Bromine melts at a lower temperature than ethanol. |
Short Answer
Which substance has the lowest boiling point?
Which substance has the highest melting point?
If temperature were increased at the same rate for all four substances, which substance would turn into a gas first?
If ethanol melts at –115 °C, at what temperature does it freeze?
Which substance has to be the coolest before it starts condensing?
There are 100 degrees between water's melting and boiling points. How many degrees are there between ethanol's melting and boiling points?
If the temperature were heat up from -10 to 65, which substance would be most reactive?
One goal of this activity was for you to see that every pure substance has its own unique properties. How does your completed diagram help you to see this?
Is changing a substances state of matter (phase change) a physical change or a chemical change? Explain.
Suppose your teacher gives you a container of salt water and a container of water. Neither of the containers is labeled. Your teacher tells you, your job is to determine which container is salt water and which container is water. You’re not allowed to taste the substances, and both containers of liquid look exactly the same. Use your knowledge of physical properties and the phase changes to explain what you could do to determine which container is water.
Name________________________________________ CP _____ Date_______
Melting and Boiling Point Investigation
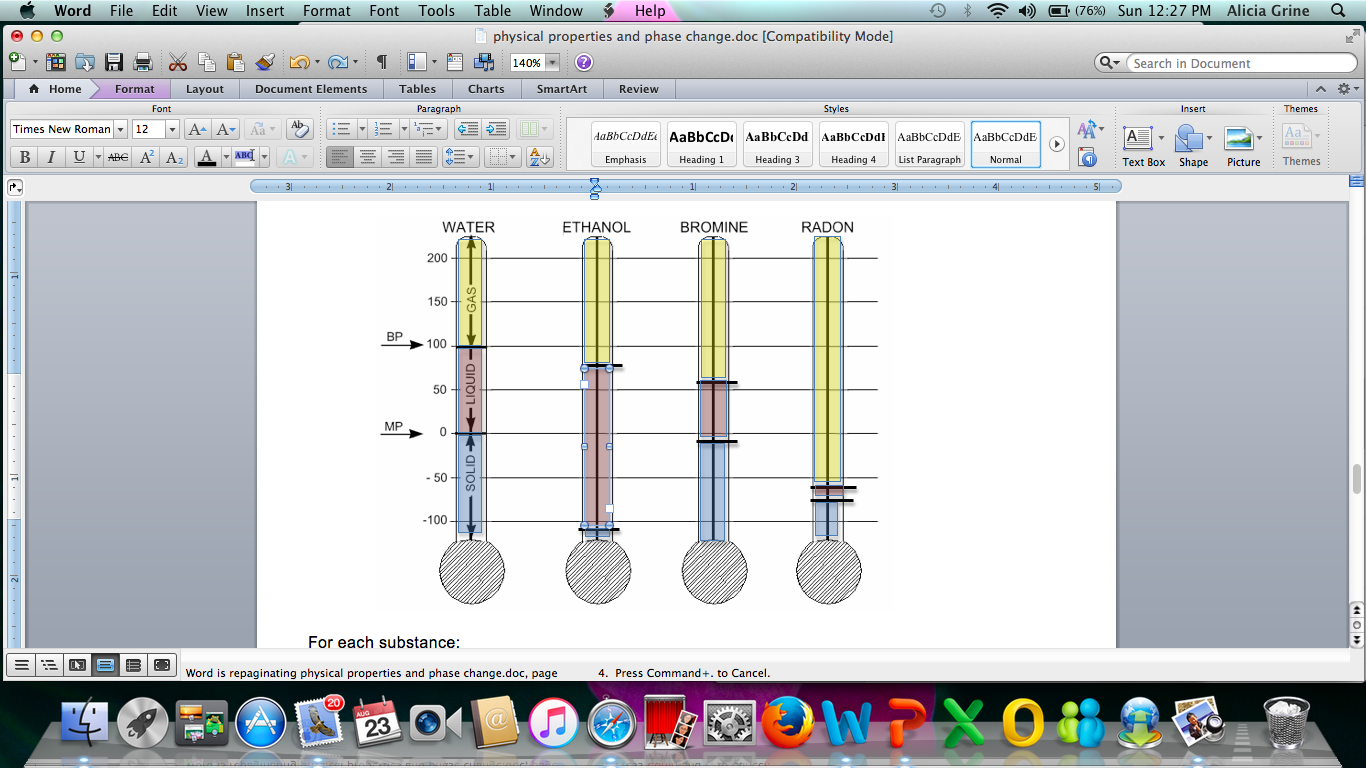
Color the thermometer graphs according to the instructions at the bottom of this page. Some of the information has already been put onto the “Water” graph for you. Then, answer the questions on the back of this page.
|
Substance |
Melting
Point |
Boiling
Point |
|
Water |
0 |
100 |
|
Ethanol |
–115 |
78 |
|
Bromine |
–7 |
59 |
|
Radon |
–71 |
–61 |
For each substance:
Use a pencil to darken the melting point and boiling point lines.
With a yellow pencil, color in the area of the thermometer directly above the boiling point line.
With a red pencil, color in the area of the thermometer between the boiling point and melting point lines.
With a blue pencil, color in the area of the thermometer below the melting point line.
Use a pencil to add arrows to indicate the melting and boiling points. Place the arrows to the left of each thermometer and label them "BP" and "MP."
In each of the yellow regions, write the word gas.
In each of the red regions, write the word liquid.
In each of the blue regions, write the word solid.
True or False
True |
False |
|
|
Bromine is a gas at – 60 °C. |
✓ |
|
|
Radon is a solid at – 100 °C. |
✓ |
|
|
Ethanol is a gas at 140 °C. |
✓ |
|
|
Water is a liquid at – 5 °C. |
✓ |
|
|
Bromine is a solid at 0 °C. |
✓ |
|
|
Radon melts at a lower temperature than water. |
✓ |
|
|
Bromine melts at a lower temperature than ethanol. |
✓ |
Short Answer
Which substance has the lowest boiling point? Radon
Which substance has the highest melting point? Water
If temperature were increased at the same rate for all four substances, which substance would turn into a gas first? Radon
If ethanol melts at –115 °C, at what temperature does it freeze? -115, because melting point and freezing point are the same.
Which substance has to be the coolest before it starts condensing? Radon
There are 100 degrees between water's melting and boiling points. How many degrees are there between ethanol's melting and boiling points? 193
If the temperature were heat up from -10 to 65, which substance would be most reactive? Bromine
One goal of this activity was for you to see that every pure substance has its own unique physical properties. How does your completed diagram help you to see this? Melting point and boiling point are physical properties. This activity shows that each of the 4 elements have different melting and boiling points.
New substances form as a result of a chemical change. Based on this knowledge is changing a substances state of matter (phase change) a physical change or a chemical change? Explain. Phase change is a physical change because nothing new is formed. The substance just changes its state of matter.
Suppose your teacher gives you a container of salt water and a container of water. Neither of the containers is labeled. Your teacher tells you, your job is to determine which container is salt water and which container is water. You’re not allowed to taste the substances, and both containers of liquid look exactly the same. Use your knowledge of physical properties and the phase changes to explain what you could do to determine which container is water. You could boil both substances. Only the water will boil at 100 degrees Celsius because boiling at 100 degrees Celsius is a physical property of water. Salt Water is a different substance so it will have a different boiling point.
1 FULTON PATSY PARCEL 043001200006009002 PHYSICAL LOCATION COUNTY
10 IN C GILLETT & B LOEWER EDS PHYSICALISM
103 DISABILITY IN THE MIDDLE AGES REPRESENTATIONS OF PHYSICAL
Tags: boiling point, different boiling, properties, standard, essential, melting, boiling, physical, point
- PROMT TRANSLATION SOFTWARE PROMT PROFESSIONAL 80 DESCRIPCIÓN BREVE
- LIGJ NR9047 DATE 1072003 PER SHERBIMIN USHTARAK NE REPUBLIKEN
- VEDLEGG 8 DEFINISJONSKATALOG AKTIVITETSUTVIKLING FOR Å
- 4 ПЕРЕЛІК ТЕХНІЧНИХ ЗАСОБІВ ЯКІ МОЖУТЬ ЗАСТОСОВУВАТИСЯ В ТЕЛЕКОМУНІКАЦІЙНИХ
- ASSESSMENT AND ASSURANCE OF INDOOR ENVIRONMENT QUALITIES IN PROGRAM
- GEOGRAPHY 345 SPRING 2003 ESSAY SCORING GUIDE NOTE THIS
- VACCINE SAFETY VACCINATION IS ONE OF THE MOST EFFECTIVE
- SALEM ACADEMY & COLLEGE VEHICLE USE POLICY EFFECTIVE AUGUST
- N HS PENSIONS NEW EMPLOYEE QUESTIONNAIRE AS PART
- ACUERDO 922020 DE 3 DE DICIEMBRE DE LA JUNTA
- LOS 20 NUEVOS QUIROPRÁCTICOS DEL CENTRO CATALÁN SE UNEN
- ASTILLERO TECNAO SA RESEÑA HISTÓRICA INICIA SUS TAREAS EL
- ACUERDO DE TRANSFERENCIA DE MUESTRAS O TEJIDOS HUMANOS POR
- SCHOOL DISTRICT BOUNDARIES FOR THE CITY OF LONSDALE LEGEND
- ACUERDO POR EL QUE SE ESTABLECEN UNA SERIE DE
- WHERE SHOULD YOU LOOK FOR DIAMONDS? AS A GEOLOGIST
- ANPS 019 BENEYTOSANTONJA 113012 SPECIAL SENSES II AUDITORY
- ACUERDOS Y CONCLUSIONES ADOPTADOS POR EL COMITÉ NACIONAL DEL
- AGENCY NAME FRPM SITE FAMILY IDENTIFICATIONCASE NO INITIAL
- ANNEX1 APPLICATION PROCEDURES OF THE CHINESE GOVERNMENT SCHOLARSHIP (20132014
- ZAŁĄCZNIK NR 2 DO REGULAMINU USTALANIA WYSOKOŚCI PRZYZNAWANIA I
- ATOS E EVENTOS TABELA RESUMIDA COM A INDICAÇÃO DOS
- ACTIVIDADES APLICACIÓN DE PORCENTAJES I ALIMENTOS 1 U N
- GUÍA DE AUTOEVALUACIÓN PARA CARRERAS DE ARQUITECTURA SISTEMA ARCUSUR
- ACUERDO DE ENCOMIENDA DE GESTIÓN ENTRE EL MINISTERIO DE
- INFORME RAONAT SOBRE LA IDONEÏTAT DEL MEMBRE PROPOSAT PER
- ZAŁĄCZNIK DO UCHWAŁY NR KEXVI00071512016 RADY GMINY DĘBE WIELKIE
- KAFKAS ÜNİVERSİTESİ ATATÜRK SAĞLIK HİZMETLERİ MESLEK YÜKSEKOKULU STRATEJİK PLAN
- 4 SECTION VIII PLAN GENERAL PROVISIONS
- İHKİB KAĞITHANE MESLEKİ VE TEKNİK ANADOLU LİSESİ TEKSİL TEKNOLOJİ
XXI SUBIDA A LA SANTA DE TOTANA 2223
RESPECTO AL REGISTRO DE RESIDUOS PELIGROSOS QUE DEBE DE
ANFANG DES 16 JAHRHUND SPIELSZENEN ZUR EINFÜHRUNG IN
 Courts of British Columbia Logo bc Flag Home
Courts of British Columbia Logo bc Flag Home KUPNÍ SMLOUVA UZAVŘENÁ PODLE § 2079 A NÁSL ZÁKONA
KUPNÍ SMLOUVA UZAVŘENÁ PODLE § 2079 A NÁSL ZÁKONAADVANCED INTERAGENCY CONSULTATION PILOT FINAL DRAFT STUDY GUIDE FOR
Once Upon the Time There Lived a Fisherman and
 EXPTE 14617 INGRESO 080620 HORA 1922 PROYECTO DE RESOLUCION
EXPTE 14617 INGRESO 080620 HORA 1922 PROYECTO DE RESOLUCION APPLICATION CRITERIA 1417 YEARS OLD SAFETY PLANS
APPLICATION CRITERIA 1417 YEARS OLD SAFETY PLANS REPÚBLICA ARGENTINA PODER EJECUTIVO NACIONAL 2018 AÑO
REPÚBLICA ARGENTINA PODER EJECUTIVO NACIONAL 2018 AÑOPATVIRTINTA ŠVENČIONIŲ JULIAUS SINIAUS MENO MOKYKLOS DIREKTORIAUS 2020 M
DEAR EDITOR REFERENCE IS MADE TO A SEPT 5TH
 REPUBLIKA HRVATSKA DUBROVAČKONERETVANSKA ŽUPANIJA GRAD OPUZEN GRADONAČELNIK OPUZEN
REPUBLIKA HRVATSKA DUBROVAČKONERETVANSKA ŽUPANIJA GRAD OPUZEN GRADONAČELNIK OPUZEN EXPEDIENTE HC 200835 PLIEGO DE PRESCRIPCIONES TÉCNICAS TUBOS ENDOTRAQUEALES
 COMUNICADO DE PRENSA INDRA LIDERA EL PROYECTO DE I+D+I
COMUNICADO DE PRENSA INDRA LIDERA EL PROYECTO DE I+D+I COT CÓDIGO DE OPERACIÓN DE TRANSPORTE DESCRIPCIÓN DEL
COT CÓDIGO DE OPERACIÓN DE TRANSPORTE DESCRIPCIÓN DELNOAA – NATIONAL WEATHER SERVICEOHD SCIENCE INFUSION AND SOFTWARE
BEWERBUNG UM EINEN PRAKTIKUMSPLATZ IM RAHMEN EINER EINSTIEGSQUALIFIZIERUNG (EQ)
 POWERPLUSWATERMARKOBJECT PART 3 WARRANTLESS POWERS TO SEARCH PLACES
POWERPLUSWATERMARKOBJECT PART 3 WARRANTLESS POWERS TO SEARCH PLACES1DIAGNÓSTICO Y POLÍTICA EN LA PLANIFICACIÓN REGIONAL (ASPECTOS METODOLÓGICOS)