IN SITU HYBRIDIZATION USING FROZEN SECTIONS DAY 1
9 NONRADIOACTIVE IN SITU HYBRIDIZATION OF PARAFFINAN INTRODUCTION TO COMPARATIVE GENOMIC HYBRIDIZATION JENNIFER LAUDADIO MD
APPENDIX FIG 2 HYBRIDIZATION PATTERNS OBTAINED FOR ECOR I
IN SITU HYBRIDIZATION BASED ON R ESCALANTE AND WF
IN SITU HYBRIDIZATION USING FROZEN SECTIONS DAY 1
JOHNSON LAB IN SITU HYBRIDIZATION PROTOCOL (ON FROZEN SECTIONS)
In situ hybridization using cryostat sections
In situ Hybridization using frozen sections
Day 1
1. Warm slides for 20 min on slide warmer
2. Pre-warm Proteinase K buffer at 37C (100 mM Tris-HCl, pH 8.2, 50 mM EDTA)
3. Fix slides in 4% paraformaldehyde for 15 min
4. Rinse 3 x 5 min in 1x PBS
5. 5 min in Proteinase K (0.1 g/mL) in buffer
6. 1 min in DEPC-H20 at RT
7. 10 min in 4% paraformaldehyde
8. 5 min in 1x PBS
9. Make 0.1 M triethanolamine/acetic anhydride (200 mL DEPC-H20 plus 2.32 mL triethanolamine – add 600 L just before pouring onto slides)
10. Incubate slides 10 min with above TAE/acetic anhydride mixture
11. 3 x 5 min in 1x PBS
12. 100 L pre-hybridization solution under parafilm coverslip for 1 hr at 60C
13. Heat denature probe for 5 min at 95C, then place on ice until used
14. 100 L pre-hybridization solution with dilution of probe (1:100 to 1:1000) under parafilm and glass coverslip at 60C overnight
Day 2
1. Pre-heat 50% formamide, 0.5x SSC wash to 60C
2. Take NBT and BCIP out of freezer to warm up
3. Gently remove cover slips by lifting corner with a razor blade
4. Wash sections in a Coplin jar with 50% Formamide/0.5x SSC for 1 hr at 60°C
5. 0.5x SSC for 5 min at RT
6. Incubate with 100 L antibody blocking solution under a parafilm cover slip for 1 hr at RT. This should be done in a humidified tupperware box on a blue pad modestly wetted with H2O to prevent drying.
7. Prepare a 1:1000 dilution of anti-DIG-alkaline phosphatase in blocking solution. This is another step that may need to be fine-tuned.
8. Gently remove cover slip with a razor blade.
9. Incubate with 100 L diluted anti-DIG-alkaline phosphatase (Fab fragments) under a parafilm cover slip for 1 hr at RT. This should be done in a humidified tupperware box on a blue pad modestly wetted with H2O to prevent drying.
10. Remove cover slips with razor blade and wash 2 x 5 min in 1x PBS
11. 3 x 10 min in chromagen buffer.
Chromagen buffer: 100 mM Tris-HCl (pH 9.5), 100 mM NaCl, 50 mM MgCl2. Note: It is VERY important to make this fresh for each experiment as it will absorb CO2 and change pH over time
12. Prepare the chromagen solution and keep in the dark:
For each 50 ml Coplin jar: 50 ml chromagen buffer, 225 L NBT, 175L BCIP
Incubate slides in chromagen solution from 2-24 hr, as needed (try going overnight first). Wrap Coplin jar in foil and store in a dark place.
Day 3
1. When color development is optimal, wash 10 min in 1x PBS.
2. Fix 10 min in 4% paraformaldehyde
3. 5min PBS.
4. 5min 95% EtOH
5. 5min 100% EtOH
6. 2x5min xylene
7. coverslip with Permount
MICROARRAY HYBRIDIZATION USING CY3LABELED RANDOM 9MERS 1 REHYDRATE SLIDE
Slide Processing Hybridization Washing and Particle Binding Protocol for
SUPPLEMENTARY FIGURE LEGENDS FIGURE S1 WHOLEMOUNT IN SITU HYBRIDIZATION
Tags: frozen sections, frozen, using, hybridization, sections
- CASO ISLA DE PALMAS – FALLO ARBITRAL 1928 CASO
- UCD COLLEGE OF ENGINEERING & ARCHITECTURE MODULE REGISTRATION FOR
- POLITECHNIKA WROCŁAWSKA WYDZIAŁOWY ZAKŁAD INŻYNIERII LOTNICZEJ NA WYDZIALE MECHANICZNOENERGETYCZNYM
- STARRING ANTHONY HOPKINS AS PURE EVIL! AN ANALYSIS OF
- 5 RENTA – ACTUAL LEY SOBRE IMPUESTO A LA
- BASES DE DATOS Y CATÁLOGOS (SESIÓN 3ª) CADA
- DIUMENGE 14È DURANT L’ANY CICLE A ES REVELA LA
- ACTIVITATS UNITAT 1 1 1 BUSCA DIVERSES FOTOGRAFIES
- CEIP ALEGRÍA DE LA HUERTA 3º PRIMARIA NATURAL SCIENCE
- SHOULD A NEW ARENA BE BUILT IN PITTSBURGH? AN
- 26082016 DEPORTES ACUATICOSWATER SPORTS ESCUELA DE BUCEO
- MODELO 6 ACTA DE RECEPCIÓN DE EDIFICIO
- MINISTÉRIO DA EDUCAÇÃO UNIVERSIDADE FEDERAL DA INTEGRAÇÃO LATINOAMERICANA PLANO
- โรคไข้เลือดออกในผู้ป่วยเด็กรพสงขลานครินทร์ พศ 2544 ปุณณดา สุไลมาน โรคไข้เลือดออกเป็นโรคติดเ
- “ŠTO SE TIČE DREVNOSTI INDIJSKE MUDROSTI LJUDI SU UŽIVALI
- DICTAMEN Nº 2592008 TÍTULO CONSULTA Nº 2382008 SOBRE LA
- „NOC GROZY” WAMPIRY WRÓŻBY TAŃCE – NIEPRZESPANA NOC W
- ITNL ISC 2005 UNIDAD IV DIFERENCIACIÓN E INTEGRACIÓN NUMÉRICA
- UN CRIPPLE IMITA UN ADULTO DE MAYFLY QUE NO
- MF’S BÁSICO 1 COURSE AT HTTPSBLOGFORBASICO1WORDPRESSCOM CÓMO PENSAR BIEN
- AUTORIZACIÓN PARA REVELAR INFORMACIÓN DE FLORIDA KIDCARE ESTIMADO PARTICIPANTE
- ACTE NECESARE DREPTURI OBLIGATII VIZITATORI ACTE NECESARE FORMALITATILOR DE
- MOTHER GOOSE REBUS RHYMES HEY DIDDLE DIDDLE JACK
- MODELO DE CARTA DE GARANTIA HAMBURG SUD Y
- E L DIA DE LOS MUERTOS INTRODUCCIÓN ¿SABES QUÉ
- SEGMENTAL ENVIRONMENTS OF SPANISH DIPHTHONGIZATION P 29 SEGMENTAL ENVIRONMENTS
- MUESTRA “LAS COSAS QUE NO SE NOMBRAN” COLECTIVO CARACÚ
- I CCINTERNATIONAL MARITIME BUREAU (PIRACY REPORTING CENTRE) PIRACY &
- PUNKTY Z ZACHOWANIA LP RODZAJ DZIAŁANIA PUNKTY I1 UCZEŃ
- DATA WPŁYWU WNIOSKU WNIOSEK O PRZYZNANIE STYPENDIUM SZKOLNEGO
NEL MONDO INVISIBILE DI LÉON DENIS 1 PREMESSA LA
RECURSO DE QUEJA NO 43481 IRMA REYES UMAÑA CORTE
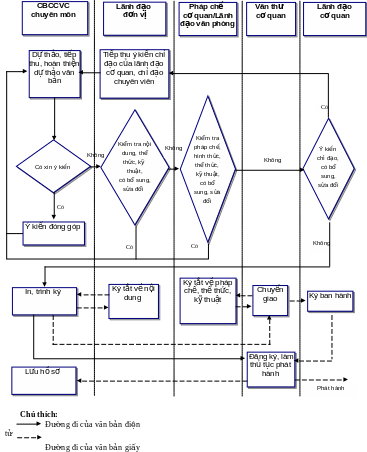 2 QUẢN LÝ VĂN BẢN ĐI TRONG MÔI TRƯỜNG
2 QUẢN LÝ VĂN BẢN ĐI TRONG MÔI TRƯỜNGREJESTR DECYZJI O ZEZWOLENIU NA REALIZACJĘ INWESTYCJI DROGOWEJ 2010
 NUESTRO PADRE NO HA DECLARADO HOY ANTE EL JUEZ
NUESTRO PADRE NO HA DECLARADO HOY ANTE EL JUEZINFORMATIKA TANMENET 6 OSZTÁLY (37 ÓRA) I INFORMATIKAI ESZKÖZÖK
 IMPLEMENTATION OF A TRAINING STRATEGY FOR INFECTION CONTROL IN
IMPLEMENTATION OF A TRAINING STRATEGY FOR INFECTION CONTROL INSouth Dakota School of Mines and Technology Department of
 “EL ROMANÍ TÉ VIRTUTS SENSE FI” RODOLÍ ANÒNIM “EL
“EL ROMANÍ TÉ VIRTUTS SENSE FI” RODOLÍ ANÒNIM “ELKVALITETSRAPPORT FOR SKOLEÅRET 200809 HØRINGSSVAR FURESØKREDSEN HAR FØLGENDE KOMMENTAR
TABLE S1 REAL SCORE OF MEAN EXPRESSION (AU) OF
UNIVERSIDAD NACIONAL AUTONOMA DE MEXICO LENGUAJES DE PROGRAMACION [JAVA
 RESTRICTED ACCOUNTS AND AFFECTED BY CHANGE ACCOUNTS ABOUT THIS
RESTRICTED ACCOUNTS AND AFFECTED BY CHANGE ACCOUNTS ABOUT THISDOCUMENTS YOU NEED TO BRING WITH YOU AT YOUR
 ILICA 219A 10000 ZAGREB OIB 97475640707 IBAN HR0723600001101359972 HRVATSKO
ILICA 219A 10000 ZAGREB OIB 97475640707 IBAN HR0723600001101359972 HRVATSKO NA OSNOVU ČLANA 22STAV 2 ZAKONA O IZVRŠAVANJU BUDŽETA
NA OSNOVU ČLANA 22STAV 2 ZAKONA O IZVRŠAVANJU BUDŽETAACCOMMODATION ANDY’S PLACE B&B 34 BOURKE ST 0415 865
 THE IONIC STYLE IONIC EVOLVED IN IONIA ON THE
THE IONIC STYLE IONIC EVOLVED IN IONIA ON THE HIRING MANAGERS GO TO WWWJOBSATOSUCOMHR TYPE IN
HIRING MANAGERS GO TO WWWJOBSATOSUCOMHR TYPE IN S OP 423 MANAGING MERCURY CONTENTS 1 INTRODUCTION
S OP 423 MANAGING MERCURY CONTENTS 1 INTRODUCTION