COPPER MINING LAB CLASS COPY BACKGROUND READING EXPERIMENT OVERVIEW
19 PREDIAGNOSTIC COPPER AND ZINC BIOMARKERS AND COLORECTAL CANCER192 COPPER PLATING A NAIL DESCRIPTION WHEN A NAIL
7 MODELLING COPPER (II) LIQUIDLIQUID EXTRACTION THE SYSTEM ACORGA
A HISTORY OF B MASON & SONS COPPERCOPPER
A UNIQUE FORM FOR COPPER INFILTRATION – WROUGHT WIRE
ANTHROPOGENIC SOURCES OF ARSENIC AND COPPER TO SEDIMENTS OF
Copper Mining Laboratory
Copper Mining Lab Class Copy
Background Reading
Experiment Overview:
The purpose of this experiment is to simulate heap leach extraction of copper from copper ore. Copper metal will be recovered using a single replacement reaction.
Introduction:
Mining is the extraction of metals or minerals from the Earth. Mining done above ground is called surface mining, and mining below ground is called subsurface mining. All types of mining pose environmental challenges. This activity focuses on the surface mining of copper.
Background:
Copper is mined from deposits of native copper (Cu2), cuprite (CuO2), azurite (Cu3(CO3)2(OH)2, and malachite (Cu2CO3(OH)2, and chalcopyrite (CeFeS2). In ancient times, nuggets of native copper were collected in streams or found lying on the ground. The native copper collected in this way was essentially pure copper metal. Simply heating these copper nuggets at a high temperature was enough to melt the metal so that it could be cast or formed into jewelry, weapons, or household objects. Native copper was also found projecting out of the ground in what is known as veins. This discovery led to surface and crude subsurface shaft mining of the ore. Surface mining involves scraping or digging to remove the layers of soil and rock that cover the vein of the metal or mineral. Subsurface mining involves digging long holes, or shafts, from an above ground entrance to very deep levels underground. Gradually, the large veins of copper have been mined to completion causing the less pure forms of copper such as the malachite and chalcopyrite to become the most common source of copper. These copper minerals are often found close to the surface making surface mining the most common form of copper mine. Some of the largest surface mines in the world are copper mines.
T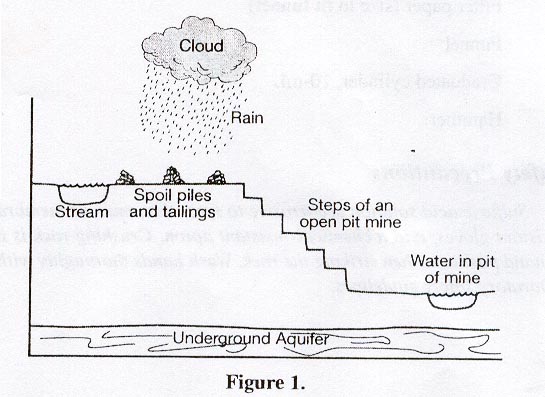 hree
steps are involved in mining – extraction of the rock, mineral
processing, and metal purification. In surface mining, the top layers
of soil and rock, called overburden or gangue, are moved away from
the ore vein and heaped into spoil piles. The ore is extracted from
the vein and moved to a processing plant. At the processing plant,
the metal ore is separated from naturally occurring non-metallic
minerals in a process called benefication. The metal ore proceeds to
the purification process while the nonmetallic waste, called
tailings, is heaped into piles similar to the spoil piles. High
quality metal ore can be initially refined by smelting. Smelting
involves heating the metal ore in a kiln like oven in a reducing
environment to “release” the copper from the nonmetal
components of the mineral.
hree
steps are involved in mining – extraction of the rock, mineral
processing, and metal purification. In surface mining, the top layers
of soil and rock, called overburden or gangue, are moved away from
the ore vein and heaped into spoil piles. The ore is extracted from
the vein and moved to a processing plant. At the processing plant,
the metal ore is separated from naturally occurring non-metallic
minerals in a process called benefication. The metal ore proceeds to
the purification process while the nonmetallic waste, called
tailings, is heaped into piles similar to the spoil piles. High
quality metal ore can be initially refined by smelting. Smelting
involves heating the metal ore in a kiln like oven in a reducing
environment to “release” the copper from the nonmetal
components of the mineral.
Ecosystems surrounding mines are often adversely affected by the tailing and spoil piles. Many of the copper minerals or surrounding minerals contain sulfur. As precipitation like rain or snow falls onto the tailing and spoil piles, the water seeps though the pieces of rock. Species of sulfur loving bacteria, water, and oxygen react to create sulfuric acid. The solubility of metals increases with decreases in pH. The sulfuric acid solution leaches metals, such as iron, copper, lead, nickel, or arsenic from the tailing and spoil piles. The metal containing sulfuric acid solution, called acid mine drainage, travels to nearby streams, ponds, lakes, and even groundwater, changing the pH of the water. Acid mine drainage can be extremely corrosive, causing tissue damage and even death to many of the plant and animal species surrounding the mine. Bedrock surrounding a stream or lake near a mine contains minerals which act to neutralize the acid environment making it basic. If the water becomes sufficiently basic (alkaline), metals will precipitate out of solution coating the bottom of the stream or lake. Bottom feeding animals ingest small amounts of the metal laden sediment. Other species may bioaccumulate the metal as they feed on the bottom feeding species or on plants that have absorbed small amounts of the metal.
The chemical process behind acids mine drainage has led to the development of a process for low quality mineral ore called heap leach extraction. In heap leach extraction, crushed tailings and spoil piles are piled into a tank or onto a plastic liner on the ground. A sulfuric acid solution is sprayed onto the pile of ore. The solution permeates through the ore pile and dissolves metals from the rock. The amount of metal recovered using the heap leach extraction process can be greatly increased by the addition of specific bacteria to the mineral pile. Acidophilic, thermophilic, or chemolithotrophic bacteria thrive in the harsh conditions created in the heap leach piles. Chemolithotrophic bacteria derive energy by oxidizing inorganic compounds such as nitrogen, sulfur, hydrogen or metals. The resulting copper sulfate solution, an acidic blue liquid, is collected into vats for refining.
Several different refining techniques are used to capture the copper from the copper sulfate solution. One of the simplest methods is to add iron metal to the solution. The following reaction occurs…
CuSO4 (aq) + Fe(s) Fe2SO4(aq) + Cu(s)
The sulfate ion has a greater affinity for iron than copper. This change is observable because copper sulfate is blue while iron sulfate is colorless. Shiny copper metal plates onto the iron, while the iron metal leaches into solution. Eventually, the copper metal builds up enough to either stop the reaction or it may fall of the iron substrate allowing the reaction to continue. This is an example of a single replacement reaction often studied in chemistry.
Copper Mining Lab Class Copy
Instructions
Materials:
Chalcopyrite (1 piece)
20 mL of 2M sulfuric acid solution H2SO4
Electronic Balance
Funnel
Filter paper
10mL graduated cylinder
Hammer
Nail
Paper towels
Sandpaper
Glass stirring rod
Test tube rack
2 large test tubes
Weighing dish or paper
Safety Precaution:
Sulfuric acid solution is corrosive to the eyes, skin, mucous membranes, and other tissues. Wear approved protective eyewear, and follow all laboratory safety guidelines! Inform your teacher of a spill immediately, and wash in eyewash or sink.
Procedure:
Closely observe a piece of your mineral sample. Record observations on you laboratory sheet.
Wrap the mineral piece in several sheets of paper towel.
Place the wrapped mineral on a hard, durable surface and pulverize the rock with a hammer into pea sized or smaller pieces.
Using a balance, weight out 1 gram of the pulverized mineral and place it into a clean test tube.
Using a graduated cylinder, measure and add 10mL of 2M sulfuric acid solution to the test tube. Observe and record the reaction between the mineral and the sulfuric acid.
Allow the sulfuric acid to leach copper from the mineral for 20 minutes, stirring occasionally with a glass stirring rod.
While waiting, use sandpaper to sand the surface of the nail. Carefully place the nail point side down into a clean test tube.
Place a funnel into the top of a clean test tube with the nail.

Fold a piece of filter paper in half, and then in half again. Tear off a small corner of the filter paper. See visual directions in figure 2.
Place the folded filter paper into the funnel.
Carefully pour the sulfuric acid – mineral mixture into the filter paper. Closely observe the drops of copper sulfate solution as it drips through the filter paper and lands on the sanded nail. Record your observations on your laboratory sheet.
Observe the color of the solution as it drips from the filter paper. Record your observations on your laboratory sheet.
Follow disposal directions given by your teacher, and complete the post lab questions.
Copper Mining Lab Name __________________ Block __
Student Worksheet
Experiment Overview:
The purpose of this experiment is to simulate heap leach extraction of copper from copper ore. Copper metal will be recovered using a single replacement reaction.
Pre-Lab Questions:
Name two types of mining. ______________________ & ____________________
How was copper acquired in ancient times?
We cannot obtain enough copper using the method from question 2 to meet the global demand of copper. Why not?
Name a few types of rocks that contain copper?
After the metal ore has been separated from other minerals like quartz, what happens to the non-metallic waste?
Which of earth’s spheres are affected by copper mining? (Look up the 4 spheres in your notes!)
Some bacteria like really harsh conditions. What conditions do acidophilic bacteria like? ________________ Thermophilic? ________________
Explain the process of heap leach extraction?
Look at the chemical formula for the reaction: CuSO4 (aq) + Fe(s) Fe2SO4(aq) + Cu(s)
What happened to the copper (Cu) and the iron (Fe)?
Data:
Data for this laboratory is qualitative. Please give a thorough description for each of the parts listed below. (draw, describe, and use details)
Chalcopyrite Observations:
Reaction Observations:
Nail and Solution Observations:
Final Solution Observations:
Post-Lab Questions:
What happened to the chalcopyrite when the sulfuric acid was added?
Based on the information provided in the background section, speculate about the metal composition of the nail. Cite any specific evidence that led you to this conclusion.
Speculate how the solution would change over time if the nail was allowed to sit in the solution overnight.
Observe someone else’s test tubes. How were the results with the two samples similar? Different?
AP CHEMISTRY NAME DATE PERIOD COPPER AMMINE COMPLEX FORMATION
APLICACION DEL COPPERCOAT 1LA PARTE SUBACUÁTICA DEL CASCO SE
APPENDIX 1 COPPER CATHODE FUTURES CONTRACT OF SHANGHAI INTERNATIONAL
Tags: background reading, the background, copper, overview, reading, mining, experiment, background, class
- ANLAGE ZUR ANTWORT AUF FRAGE 10 FRAGEBOGEN DER UNABHÄNGIGEN
- ENTREGA 11 LAS PRINCIPALES VERSIONES DE LA ESCRITURA SE
- REKLAM KURULU KARARI201 TOPLANTI 12062012 HTTPWWWGUMRUKTICARETGOVTRALTSAYFAICERIK1431539REKLAMKURULUKARARLARIHTML TÜTÜN – ALKOL
- APPENDIX 4 CAPITAL INVESTMENT PROGRAMME 201417 SUSTAINABLE DEVELOPMENT PLANNING
- TC BİNGÖL ÜNİVERSİTESİ BÖLGESEL KALKINMA ODAKLI MİSYON FARKLILAŞMASI VE
- “EXTRAORDINARY & UNIVERSAL COMMOTIONS” CHARLESTON REACTS TO THE STAMP
- QUELQUES INFORMATIONS IMPORTANTES SUR LE SERVICE INTENDANCE LA RESTAURATION
- TODD DECKER TDECKERWUSTLEDU EDUCATION 2007 UNIVERSITY OF MICHIGAN ANN
- ECTS EUROPEAN CREDIT TRANSFER SYSTEM TRANSCRIPT OF RECORDS
- OPDRACHT BANKGARANTIE INVULLEN MET MICROSOFT® WORD OPDRACHTGEVER ZEKERHEIDSTELLER
- DEPARTAMENTO BIOLOGÍA MOLECULAR E INGENIERÍA BIOQUÍMICA ÁREA ACADÉMICA
- CLASES DE REFUERZO CURSO 20172018 SON CLASES EN LAS
- 2012 COSIDA SPONSOR DIRECTORY OVER THE YEARS COSIDA HAS
- EMERGING MEASURES CONTENTS CHILD AND ADOLESCENT HEALTH MEASUREMENT
- ANEXO III HOJA DE INSCRIPCIÓN POR CENTRO ASISTENCIAL
- LULLABY FOR MY MOTHER LET ME KISS AWAY THE
- OFFICE OF THE ADVOCATE GENERAL FOR SCOTLAND DEVOLUTION ISSUES
- Razpisna Dokumentacija Priloga 1 Podrobnejša Predstavitev Ciljev po
- SVEUČILIŠTE U ZADRU ODJEL ZA IZOBRAZBU UČITELJA I ODGOJITELJA
- URTEA AÑO 2017 ESKATZAILEAREN DATUAK DATOS DELDE
- P RESENTS A SHORT FILM DIRECTED BY JUAN PABLO
- C CCECE SIGNIN SHEET ~ REGIONAL MEETING REGION
- TABULKY NÁPADU 2014 – NAD 500 DOPRAVNÍ A OBECNÉ
- CARACTERISTICAS TÉCNICAS DO EQUIPAMETNO MODELO 07 EDITAL DE
- III ENCUENTRO DIOCESANO DE JÓVENES COFRADES INSCRIPCIÓN NOMBRE COMPLETO
- PROPUESTA TRABAJO DE GRADO PARA OPTAR POR EL TÍTULO
- LICENCIA DE APERTURA IREKITZEKO LIZENTZIA EXPEDIENTE Nº ZK
- 1° ENCUENTRO ACADÉMICO “POR UNA JUSTICIA DE GÉNERO” 1
- NAME SOLUTIONS TEST 1 USE THE TERMS SOLUTE
- DISTRICT COURT MAINTENANCE APPLICATIONS IN THE DUBLIN AREA SELF
MINISTERUL EDUCATIEI NATIONALE INSPECTORATUL SCOLAR AL JUDETULUI BACAU LICEUL
PLIEGO DE CONDICIONES ADMINISTRATIVAS PARTICULARES QUE RIGE LA ENAJENACIÓN
19 TEXAS ADMINISTRATIVE CODE CHAPTER 76 EXTRACURRICULAR ACTIVITIES AS
(PROSZĘ WYPEŁNIĆ DRUKOWANYMI LITERAMI) PRUSZKÓW DNIA DANE WNIOSKODAWCY
 ALFREDAS CHEIDOKAS NUSIDĖJĖLĖ APIE KĄ NUTYLĖJO EVANGELIJA PAGAL JONĄ
ALFREDAS CHEIDOKAS NUSIDĖJĖLĖ APIE KĄ NUTYLĖJO EVANGELIJA PAGAL JONĄHÁBITOS DEPORTIVOS DE LOS ESPAÑOLES AGAPITO FLORIANO LACALLE SIEMPRE
 FOR IMMEDIATE RELEASE AIRCRAFT SPRUCE NOW CARRIES REDBIRD FLIGHT
FOR IMMEDIATE RELEASE AIRCRAFT SPRUCE NOW CARRIES REDBIRD FLIGHTANTIKES UND MODERNES RECHTSDENKEN VERTIEFUNGSVORLESUNG IM RAHMEN DES INTERDISZIPLINÄREN
«DE ROMANCERO VIEJO Y EDICIÓN DIGITAL EL EJEMPLO DE
MODULE 3 DEFINITIONS CHAMP D’APPLICATION PRINCIPES
 N YPD REWARD PROGRAMS 1 COP SHOT REWARD PROGRAM
N YPD REWARD PROGRAMS 1 COP SHOT REWARD PROGRAM(PIECZĄTKA HUFCA) …………………………… DNIA ………………2016 ROKU KOMENDA CHORĄGWI WIELKOPOLSKIEJ
MODÈLE 11 TABLEAU2 DES VERBES À L’ÉTUDE POUR CHAQUE
3 VÝVOJ POČTU NOVĚ HLÁŠENÝCH PŘÍPADŮ PRACOVNÍ NESCHOPNOSTI A
 PART I TO BE FILLED OUT BY THE
PART I TO BE FILLED OUT BY THE WAIT LIST FUNCTIONALITY EXPECTATIONS BASED ON COURSE SET UP
WAIT LIST FUNCTIONALITY EXPECTATIONS BASED ON COURSE SET UP EARLY CHILDHOOD CARIES POLICY STATEMENT ASSOCIATION OF STATE AND
EARLY CHILDHOOD CARIES POLICY STATEMENT ASSOCIATION OF STATE AND 6 TALLER DE SENSIBILIZACIÓN CULTURAL NORMAS DE PARTICIPACIÓN CUADO
6 TALLER DE SENSIBILIZACIÓN CULTURAL NORMAS DE PARTICIPACIÓN CUADOZAHTJEV ZA IZDAVANJE LICENCIJE ZAJEDNICE ZA OBAVLJANJE PRIJEVOZA TERETA
Ðïࡱáþÿ ¥ác ð¿ Wbjbj´x89´x89 8¢öãföãfänÿÿÿÿÿÿ·òò7)7)x87)x87)x87)ÿÿÿÿx9b)x9b)x9b)8ó)ô§+üx9b)lµäx83x83x83x83x83 7 7 7ë´í´í´í´í´í´í´0¸¶æºpñ´x87) 7þ6þ6