VACCINE STORAGE AND TRANSPORT INACTIVATED VACCINE VACCINE STORAGE INACTIVATED
0 SAMPLE COVID VACCINE ELIGIBILITY VERIFICATION LETTER WEANNEX 3 TEMPLATE FOR A NEW VACCINE INTRODUCTION PLAN
ANTIHER2 VACCINES NEW PROSPECTS FOR BREAST CANCER THERAPY MAHA
CALL CENTER FOR REGISTRATION FORM AND VACCINES THAT ARE
CHIKUNGUNYA VIRUS VACCINE STRAIN (18125) MINIMUM PERSONAL PROTECTIVE EQUIPMENT
CITY OF TULSA MEDICAL SECTION FLU VACCINE INFLUENZA VACCINE
Vaccine Storage and Transport
Vaccine Storage and Transport
Inactivated Vaccine
Vaccine Storage
Inactivated influenza vaccine must be refrigerated at 2° to 8°C (35° to 46°F).
Inactivated influenza vaccine must not be frozen.
If frozen, vaccine must be discarded.
Vaccine volume
Fluvirin (Novartis) – A box containing 10-10 multidose vials (100 doses) is 41.25 cubic inches (6 x 2.75 x 2.5 in)
Fluzone (Sanofi-Pasteur) A box containing 1 multi-dose vial (10 doses) is 3.71 cubic inches (1 1/4 x 1 1/4 x 2 3/8 in)
Fluzone (Sanofi-Pasteur) One box of ten pre-filled 0.5cc syringes is 43.31 cubic inches (4 1/2 x 1 3/4 x 5 1/2 in)
Transport
Inactivated influenza vaccine may be transported to clinics in an ice chest as per the directions below
Use in Clinic
Small amounts of inactivated vaccine may be drawn up into syringes from multidose vials in advance, but must be discarded if not used at the end of the day.
Partially used multidose vials may be returned to the refrigerator and used the next day.
Live, attenuated vaccine
Vaccine Storage
FluMist is now refrigerator stable.
FluMist
is
SHIPPED FROZEN and
upon receipt should be stored in the refrigerator between 35° to
46°F (2° to 8°C).
Do not refreeze FluMist.
Vaccine volume
FluMist comes in a 10 dose package with a volume of 39 cubic inches per 10 doses
(FluVirin, Novartis - 6.125 x 4.625 x 1.375 in)
Vaccine
transport
FluMist may be transported to clinic in an ice chest using cold packs as per the directions below.
Use in Clinic
Each vaccination station should have a small cool box with a cold pack and a thermometer to store a small quantity of vaccine.
A single dose of FluMist is taken out of the cool box for each child and thawed as per the instructions in the package insert.
FluMist that has been thawed must be discarded if not used within 24 hours.
FluMist that is taken out of the icebox, thawed, exposed to room temperature and not used must be discarded.
Options for Bulk Vaccine Storage
Existing cold room/refrigerators at the health department if sufficient capacity is available
Hospitals, nursing homes or pharmacies may have refrigerated storage that can be used.
A refrigerated truck or trailer could be rented.
Cold storage warehouses used for food are not permitted to store vaccine.
Temperature must be monitored in all storage facilities
Transporting influenza vaccine, both TIV and LAIV
Appropriate packing is a must to ensure the viability of the vaccine. Pack the vaccine as follows:
Use an insulated cooler, a Styrofoam cooler with 2” walls, or a hard-sided cooler.
Place refrigerated packs (not frozen) on the bottom of the cooler.
Add a couple inches of crushed paper over the refrigerated packs.
Place the vaccine on top of the paper in the center of the cooler.
Place a thermometer near the vaccine.
Add another couple of inches of crushed paper on top of and around the vaccine to make sure the vaccine does not shift.
Finish with another layer of refrigerated packs on top of the paper. Place the lid on the cooler and close securely. Label the contents as “Perishable” or “Biologics” or “Vaccines.”
If you are taking the vaccine to an off-site clinic, check and record the temperatures in the coolers at least hourly.
CONSENT FORM MENINGOCOCCAL GROUP ACWY VACCINE AND TETANUS DIPHTHERIAINACTIVATED
D EPARTMENT OF HEALTH COMMUNICABLE DISEASES PREVENTION UNIT VACCINE
DIPHTHERIA TETANUS & PERTUSSIS (DTAP) VACCINE PROTOCOL FOR CATCHUP
Tags: vaccine storage, the vaccine, vaccine, inactivated, storage, transport
- PRACTICA DE LABORATORIO FABRICACIÓN Y ANÁLISIS DE BIODIESEL DE
- 200 PHYSIO FITNESS (G) 200 BINGO (B) 300 BINGO
- EUPHA NEWSLETTER 4 2008 PUBLISHED APRIL 2008 IN
- COMUNICACIÓN Y REDES SOCIALES ABIERTA LA INSCRIPCIÓN PARA PARTICIPAR
- CELEBRACIÓN VIERNES SANTO 1 INTRODUCCIÓN 2 MONICIÓN DE ENTRADA
- PRODUCTIVITY COMMISSION GPO BOX 1428 CANBERRA CITY ACT 2601
- 6 LEÓN GUANAJUATO A 17 DIECISIETE DE ABRIL DEL
- ZICHTBARE HOUTEN STRUCTUUR + STRATEC III + GLASWOLROTSWOL +
- RADICACIÓN NO 66001310500120160022101 ACCIONANTE SANDRA NUBIA SÁNCHEZ ACCIONADO UARIV
- RENSEIGNEMENTS POUR UNE DEMANDE DE RENDEZVOUS AVEC LE PRESIDENT
- III ETAPA – PRAKTICKÉ OVĚŘENÍ VÝSLEDKŮ A JEJICH APLIKACE
- COMMUNITY COMPACTS – FOR USE IN THE COMMUNITY THIS
- AUTORIZACIÓN PARA EL USO Y LA DIVULGACIÓN DE INFORMACIÓN
- MOD 7514 REV 05 CENTRO PER L’IMPIEGO DI VIA
- ORDIN ME NR 3300 DIN 19 FEBRUARIE 2021 ORDINUL
- CALCULATOR FOR DETERMINING THE NUMBER OF COPIES OF A
- MIND IN CROYDON TERMS OF EMPLOYMENT MALE SOCIAL
- ISAÍAS 2 15 EL SEÑOR CONGREGA A TODAS
- “ELEKTRONET VI ÉVF 1 SZÁM 1997 FEBRUÁR” DR PÁPAY
- ПРОГРАМА ПІДГОТОВКИ ДО РІЧНОГО ОЦІНЮВАННЯ З ХІМІЇ ЗА КУРС
- LATEIN 2 SCHEIN KLAUSUR (WAHRSCHEINLICH TEXT VON
- 1 BRIEF DESCRIPTION OF TECHNOLOGY S PRINGS
- ILYEN EGYSZERŰ AZ ELEKTROGRAVITÁCIÓ! AZ ELEKTROGRAVITÁCIÓ TITKA FÉLELMETESEN EGYSZERŰ
- VOLUMEN 1 SECCIÓN 4 CUESTIONARIOS CUESTIONARIOS 461 A 9
- FORMULAIRE DE COMMUNICATION DE RENSEIGNEMENTS CONCERNANT LE GROUPE DE
- CONVENI DE COL·LABORACIÓ DE PRÀCTIQUES EXTERNES D’ESTUDIANTS DE LA
- ATHLETES AGAINST HUNGER THE KNIGHTS CODE OF CHIVALRY STATES
- FORMULARUL A FIŞA PROPUNERII DE ORGANIZARE A MANIFESTĂRII ŞTIINŢIFICE
- UCHWAŁA NR XVIII1822012 RADY MIASTA MŁAWA Z DNIA 29
- ARE YOU CARING FOR SOMEONE WITH ALZHEIMER’S DISEASE OR
15 MICHAEL RUF SPURE VON JOHANNES VOM KREUZ IM
8 JUAN J GARCÍA PELLICER JOSÉ V GARCÍA JIMÉNEZ
 PÁGINA 18 DE 18 SISTEMATIZACIÓN DE LA EXPERIENCIA EN
PÁGINA 18 DE 18 SISTEMATIZACIÓN DE LA EXPERIENCIA ENSUDĖTI SKYRYBOS ŽENKLUS IR ĮRAŠYTI PRALEISTAS RAIDES ATSAKYTI Į
APPENDIX B MASSACHUSETTS CIVIC LEARNING AND ENGAGEMENT ASSESSMENT FRAMEWORK
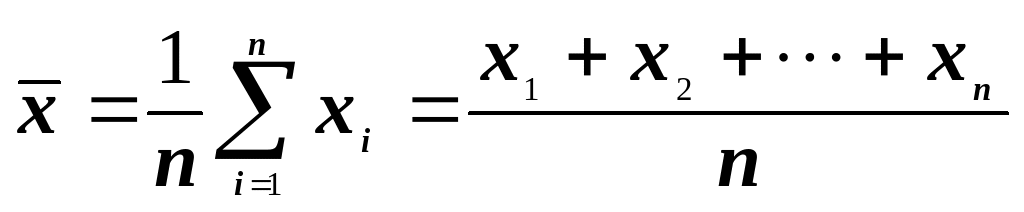 REVIEW OF BASIC STATISTICAL CONCEPTS THE PURPOSE OF THIS
REVIEW OF BASIC STATISTICAL CONCEPTS THE PURPOSE OF THIS INSTITUCIÓN EDUCATIVA JOSE ACEVEDO Y GOMEZ TALLER DE REPASO
INSTITUCIÓN EDUCATIVA JOSE ACEVEDO Y GOMEZ TALLER DE REPASOANÁLISIS SOBRE 26 ENCUESTAS RECIBIDAS ENCUESTA SOBRE PREFERENCIAS
 OSNOVNA ŠOLA STANETA ŽAGARJA LIPNICA J EDILNIK PONEDELJEK 2
OSNOVNA ŠOLA STANETA ŽAGARJA LIPNICA J EDILNIK PONEDELJEK 2TRIBUNAL PAEU 2011 CONVOCATORIAS DE JUNIO Y SEPTIEMBRE SECCIÓN
DEPARTMENT OF THE INTERIOR DEPARTMENTAL MANUAL EFFECTIVE DATE 122696
 F UNGAL DIVERSITY JOURNAL SUBSCRIPTION (ISSN 15602745) DEAR ORDERING
F UNGAL DIVERSITY JOURNAL SUBSCRIPTION (ISSN 15602745) DEAR ORDERING10 LEÓN GUANAJUATO A 31 TREINTA Y UNO DE
 Northern Maine Community College 33 Edgemont Drive Presque Isle
Northern Maine Community College 33 Edgemont Drive Presque IsleOBČINA ZREČE CESTA NA ROGLO 13B ZREČE RAZPISNA
 COMMONWEALTH OF KENTUCKY ENERGY AND ENVIRONMENT CABINET MATTHEW
COMMONWEALTH OF KENTUCKY ENERGY AND ENVIRONMENT CABINET MATTHEW APRUEBAN DIRECTIVA QUE ESTABLECE LINEAMIENTOS PARA LA PRESENTACIÓN DEL
APRUEBAN DIRECTIVA QUE ESTABLECE LINEAMIENTOS PARA LA PRESENTACIÓN DELELLEN HØRUP BREVSKRIVERLISTE BREVSKRIVERLISTE FRA CATHINKA OLSEN TIL ELLEN
ADENOVIRAL REFERENCE MATERIAL WORKING GROUP BID SUBMISSION FORM SHORTTERM
 LAMPIRAN IB ASERSI TIM KAMPANYE TINGKAT PROVINSIKABUPATENKOTA PASANGAN CALON
LAMPIRAN IB ASERSI TIM KAMPANYE TINGKAT PROVINSIKABUPATENKOTA PASANGAN CALON