AP CHEMISTRY NAME PERIOD DATE 11
17TH CHEMISTRY OLYMPIAD OF THE BALTIC STATES RIGA LATVIA1800AJ VHFREED CHEMISTRY OF HERBICIDES & PESTICIDES AND THEIR
19 FULL PAPER AUSTRALIAN JOURNAL OF CHEMISTRY TOWARDS THE
1ST 9 WEEKS PREIB PREAP CHEMISTRY FALL 2011
1ST INTERNATIONAL DAYS OF ORGANOMETALLIC CHEMISTRY AND CATALYSIS JICOC
2 CHEMISTRY 505 ALLIED HEALTH CHEMISTRY II SYLLABUS SUMMER
PS.Ch6.Test.95
AP Chemistry Name ________________________________
Period ___ Date ___/___/___
11 • IMF’s, Liquids, and Solids
B O I L I N G P R A C T I C E
Vapor Pressures of Various Liquids
|
|
|
|
|
|
|
|
Temperature (°C) |
Ethyl Alcohol (mmHg) |
Benzene (mmHg) |
Methyl Salicylate (mmHg) |
Water (mmHg) |
Carbon Tetrachloride (mmHg) |
|
–10 |
5.6 |
19 |
|
2.1 |
15 |
|
–5 |
8.3 |
25 |
|
3.2 |
20 |
|
0 |
12.2 |
33 |
|
4.6 |
27 |
|
5 |
17.3 |
43 |
|
6.5 |
35 |
|
10 |
23.6 |
56 |
|
9.2 |
45 |
|
15 |
32.2 |
71 |
|
12.8 |
58 |
|
20 |
43.9 |
91 |
|
17.5 |
74 |
|
25 |
59.0 |
114 |
|
23.8 |
94 |
|
30 |
78.8 |
143 |
|
31.8 |
118 |
|
35 |
103.7 |
176 |
|
42.2 |
147 |
|
40 |
135.3 |
216 |
|
55.3 |
182 |
|
45 |
174.0 |
263 |
|
71.9 |
225 |
|
50 |
222.2 |
317 |
|
92.5 |
271 |
|
55 |
280.6 |
379 |
|
118.0 |
325 |
|
60 |
352.7 |
451 |
1.41 |
149.4 |
389 |
|
65 |
448.8 |
531 |
1.90 |
190.0 |
462 |
|
70 |
542.5 |
622 |
2.52 |
233.7 |
547 |
|
75 |
666.1 |
720 |
3.40 |
289.1 |
643 |
|
80 |
812.6 |
843 |
4.41 |
355.1 |
753 |
|
85 |
986.7 |
968 |
5.90 |
433.6 |
877 |
|
90 |
1187 |
1122 |
7.63 |
525.8 |
1020 |
|
95 |
1420 |
1270 |
9.93 |
633.9 |
1180 |
|
100 |
1693.3 |
1463 |
12.8 |
760.0 |
1360 |
Ex1. A beaker of benzene (C6H6) is at room temperature (20° C). What is its vapor pressure? ______mmHg
Is this vapor pressure large enough to cause the benzene to boil at sea level? _____
Ex2. All of the liquids are heated to 80°C, circle those that will be boiling when the air pressure is 760 mmHg.
ethyl alcohol benzene methyl salicylate water carbon tetrachloride
Ex3. If the temperature remains unchanged, but the pressure above the liquids is reduced to 700 mmHg, circle those substances that will be boiling.
ethyl alcohol benzene methyl salicylate water carbon tetrachloride
Problems:
1. A beaker of ethyl alcohol at room temperature (20° C) and a beaker of benzene at room temperature are both placed on a hot plate on a day when the air pressure is only 700 mmHg. Which one of the two liquids will start to boil first? ________________ … It will boil at about what temperature? _________
2. A beaker of carbon tetrachloride is warmed to 60° C and connected to a vacuum pump so the air pressure above the liquid can be changed. The pump is turned on. At what pressure will the liquid start to boil? ______mmHg
What would this pressure be in atmospheres? ______ atm [show calculation here]
3. At 75° C, in a place where the air pressure is only 650 mmHg, circle the substances that will be boiling.
ethyl alcohol benzene methyl salicylate water carbon tetrachloride
4. The pressure above the five substances is adjusted to 550 mmHg. What is the approximate boiling point of each substance at this pressure? [methyl salicylate is excluded]
ethyl alcohol benzene water carbon tetrachloride
_____ _____ _____ _____
5. At 550 mmHg, when the temperature is hot enough to get the ethyl alcohol to boil, will the carbon tetrachloride also be boiling? ____
6. At 75° C, I start the pressure above the liquids at 760 mmHg and slowly reduce it using the vacuum pump. In which order will the substances boil?
1st__________ 2nd__________ 3rd__________ 4th__________ 5th__________
7. Determine the approximate “normal boiling point” of each substance below:
ethyl alcohol benzene water carbon tetrachloride
_____ _____ _____ _____
8. Study the chart of vapor pressures at 25° C. Rate the five substances in terms of their IMF’s (intermolecular forces of attraction).
strongest IMF’s ____________________
____________________
____________________
____________________
weakest IMF’s ____________________
9. Why doesn’t data for methyl salicylate start until 60° C? _____________________________________
_____________________________________________________________________________
20 SEPTEMBER 2005 G436 BIOGEOCHEMISTRY ASSOC PROF
2005 CHEMISTRY SELFAUDIT CHECKLIST PAGE 1 OF 2 DATE
2005 CHINAJAPANKOREA SYMPOSIUM ON ENVIRONMENTAL ANALYTICAL CHEMISTRY SEPTEMBER 1
Tags: chemistry name, chemistry, period
- 8 CONFOUNDS THREATEN INTERNAL VALIDITY 1) HISTORY ALL
- KLASA III WĘGLOWODORY METAN – GŁÓWNY SKŁADNIK GAZU ZIEMNEGO
- R OVENT 2012! COME JOIN US IN THIS
- ATTACHMENT A – AUTHORIZED PROVIDER SERVICES ENROLLMENT 1915(C) HCBS
- VALPARAÍSO JUEVES 05 DE MARZO 2020 CIRCULAR Nº 01
- SPECIMEN SINGLE BREED OPEN SHOW SCHEDULE THIS DOCUMENT IS
- INSTRUCCIÓN GENERAL NÚMERO 052011 A FISCALES DISTRITALES Y DE
- HAVE YOU EVER WONDERED HOW IT’S POSSIBLE TO FORMAT
- CAN WE SHOW THAT ONLY THE GREEN PARTS OF
- KARTA PRACY TEMAT 13 BUDŻET KIESZONKOWY PROSTE OBLICZENIA W
- P LEASE FILL OUT SIGN AND RETURN PROMPTLY TO
- JAMARSKA ZVEZA SLOVENIJE LEPI POT 6 PP 2544 1109
- APPLICATION FOR AFTER SERVICE HEALTH INSURANCE AND PENSION FUND
- CASE 15 A SILENT WORLD FED UP WITH THE
- BRF ENGELHOLM HTTPSWWWHSBSEOSTRAENGELHOLM INFOBLAD 1 2018 BESÖK VÅR HEMSIDA
- diana-thomas
- CARAVAN SITE LICENCE CARAVAN SITES AND CONTROL OF DEVELOPMENT
- D IVENDRES 22 DE JULIOL DE 2011 PREMIS CATALUNYA
- Nº PROCEDIMIENTO 180087 CÓDIGO SIACI SKI3 ANEXO II SOLICITUD
- CHILOE 1963 CASTRO CUECA CON ACORDEÓN ANCUD LEOCADIA MARIN
- NAJČEŠĆA PITANJA I ODGOVORI 1 TREBAJU LI SE SVE
- NEWS RELEASE 13TH AUGUST BIRMINGHAM SCHOOLS GET READY TO
- LIITE P 1 MUURAMEN KUNTA YMPÄRISTÖLUPAPÄÄTÖS TEKNISTEN PALVELUIDEN LAUTAKUNTA
- EK4 TANITIM DESTEĞİ İÇİN İBRAZ EDİLMESİ GEREKEN BİLGİ VE
- IDENTIFICACIÓN DEL INTERESADOA NIF CIF 1ER APELLIDO 2º
- PRIMORSKOGORANSKA ŽUPANIJA ZAPISNIK SA SASTANKA SAZIVAČ IME I PREZIME
- GLOBAL JRA WEB SERVICES SERVICE INTERACTION PROFILE VERSION 10
- HOGAR NAVARRO C PORTALES 23 1º LOGROÑO TELÉFONO
- U NITED NATIONS SYSTEM STANDING COMMITTEE ON NUTRITION
- OPLEGNOTITIE REGIEGROEP LBOW AGENDAPUNT 55 HELPDESK WATER 1 OPSTELLERCONTACTPERSOON
 CULTURA – PRÁTICA SOCIAL COMO OBJETO DE INVESTIGAÇÃO CULTURE
CULTURA – PRÁTICA SOCIAL COMO OBJETO DE INVESTIGAÇÃO CULTURE PN 02403 + Z1 + Z2 + Z3 HŘEBÍČEK
PN 02403 + Z1 + Z2 + Z3 HŘEBÍČEKCOA LIBRARY FUNDING SOURCES 20022019 COLLECTION EXPENDITURES BY RESOURCE
 OTÁZKY KE ZKOUŠCE Z EKONOMIE OTÁZKY JSOU ZDE PROMÍCHÁNY
OTÁZKY KE ZKOUŠCE Z EKONOMIE OTÁZKY JSOU ZDE PROMÍCHÁNYCATALUÑA COMO PROBLEMA EN LA HISTORIA DE ESPAÑA CONFERENCIA
 CONSTANCIA DE INGRESOS ECONOMICOS COMITÉ TÉCNICO DEL PROGRAMA NACIONAL
CONSTANCIA DE INGRESOS ECONOMICOS COMITÉ TÉCNICO DEL PROGRAMA NACIONALHÜ REKTÖRÜ PROF DR A MURAT TUNCER VE ÖĞRENCILER
 IES BENJAMÍN RÚA CONSEJERÍA DE EDUCACIÓN JUVENTUD Y DEPORTE
IES BENJAMÍN RÚA CONSEJERÍA DE EDUCACIÓN JUVENTUD Y DEPORTEZASADY DOKONYWANIA OCENY FORMALNEJ ZARYSÓW PROJEKTÓW I KOMPLETNYCH PROPOZYCJI
UCHWAŁA NR XIX1762004 RADY MIEJSKIEJ W WOŁCZYNIE Z DNIA
 M UĞLA SITKI KOÇMAN ÜNİVERSİTESİ AVRUPA KREDİ TRANSFER SİSTEMİ
M UĞLA SITKI KOÇMAN ÜNİVERSİTESİ AVRUPA KREDİ TRANSFER SİSTEMİFINAL CONTINUOUS LIGHTING PERMIT APPLICATION WISCONSIN DEPARTMENT OF TRANSPORTATION
DISRUPTIVE PHYSICIAN PATIENTS IDENTIFY BEING CONFIDENT EMPATHETIC HUMANE PERSONAL
 APA (AMERICAN PSYCHOLOGICAL ASSOCIATION) ¿QUÉ ES APA? NOMBRE AMERICAN
APA (AMERICAN PSYCHOLOGICAL ASSOCIATION) ¿QUÉ ES APA? NOMBRE AMERICANIII JORNADAS DE LA SOCIEDAD PARA EL ESTUDIO DEL
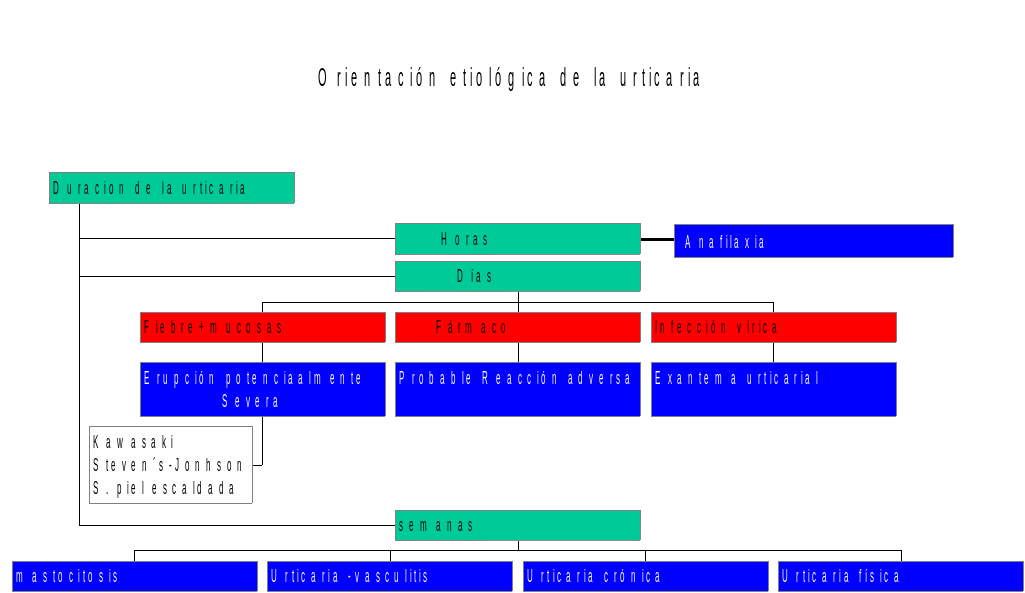 Orientación Etiológica la Urticaria dra a Bilbao Hospital de
Orientación Etiológica la Urticaria dra a Bilbao Hospital de L EY DE REMUNERACIONES DE LOS SERVIDORES PÚBLICOS DEL
L EY DE REMUNERACIONES DE LOS SERVIDORES PÚBLICOS DELRÚBRICA GENERAL CONOCIMIENTO DEL MEDIO SOCIAL NATURAL Y CULTURA
ORDINANZA RU 2014 NICHT LÖSCHEN BITTE !!
 A PPUNTAMENTO OGNI 3° SABATO DEL MESE 16 MAGGIO
A PPUNTAMENTO OGNI 3° SABATO DEL MESE 16 MAGGIO