BASIC WESTERN BLOT PROTOCOL P4EBP1 061008 BUFFER SOLUTIONS RUNNING
Consultation on dft Basic Carbon Tool for Local!DOCTYPE HTML HTML LANGEN CLASSNOJS HEAD ! BASIC
(NAME OF FACILITY) C HILD CARE EMERGENCY BASIC EMERGENCY
0 PROGRAMA BIBLIOGRAFIA BASICA UNIDAD 1 1 SELECCIÓN DE
1 BASIC CONCEPTS IN THE TRANSMISSION OF COMMUNICABLE DISEASES
1 BASIC SYNTACTIC STRUCTURES POLISH AND ENGLISH SIMPLE
Basic Western Protocol
Basic Western Blot Protocol
p-4EBP1
06/10/08
Buffer Solutions:
Running Buffer:
100 ml 10x TRIS/Gly/SDS
900 ml dH2O
Transfer Buffer:
100 ml 10x TRIS/Gly (40 ml of 25x and 760 dH2O)
700 ml dH20
200 ml methanol
TBS-T:
100 ml 10x TBS
900 ml dH2O
1 ml Tween 20
Blocking Buffer/Sln: 5% milk in TBS-T
3.5 g dry milk
70 ml TBS-T (100 ml TBS; 900 ml H20; 1 ml Tween)
Protocol:
I. Run gel:
Prepare samples for loading:
Add 2x SDS loading buffer to sample at 1:1 (only if sample not already in SDS)
Heat on heating block (100) for 5 minutes
Use pre-made gel (in 4 behind Ling’s bench)
Remove tape, mark the bottom of wells with black marker for better visualization, and remove the comb (slowly so as not to break wells)
Load gel in gel box and lock into place
Fill inner chamber of gel box with running buffer … look for leaks
Fill outer chamber ½ way or more
Add 10 ul of water to protein standard to resuspend then load (12-15 ul) into well
Load samples (max for a 10 well gel is 37 ul per well)
|
Lane # |
Cell |
Protein Number |
Total vol. loaded (uL sample, water, SDS) |
|
1 |
Rainbow |
n/a |
10 |
|
2 |
Positive Control K7M2 |
? |
25 |
|
3 |
Cardiac |
4.904 |
25 |
|
4 |
Liver |
9.378 |
14.4 |
|
5 |
Lung |
9.195 |
13.1 |
|
6 |
Spleen |
11.891 |
28.7 |
|
7 |
Kidney |
3.199 |
37.5 |
|
8 |
Pancreas |
6.563 |
Too small-40 |
|
9 |
Large Intestine |
1.883 |
40 |
|
10 |
SDS Buffer |
|
20 |
Let gel run at 120V for 1-1.5 hrs.
II. Transfer to Nitrocellulose membrane
Soak red/black transfer block and two sponge sheets in transfer buffer
Prepare nitrocellulose membrane sandwich:
Mark side of the nitrocellulose membrane that will contact gel with special marker (Ling’s bench)
Soak membrane and sandwich paper (x2) in transfer buffer
Remove gel from plastic panel, cut bottom just above protein front and at top just below the wells with ruler edge
Make Transfer “Sandwich”: black on bottom, 1 sponge, 1 piece of sandwich paper, gel, nitrocellulose membrane (marked side down), 1 piece of sandwich paper, 1 sponge, red side on top
Place the red/black transfer block in the Owl transblot chamber
Chamber should have stir bar in the bottom and be connected to the water coolant system next to it
Black side facing black, red facing red (towards the wall)
Fill chamber with transfer buffer to cover top of red/black transfer block
Run at 300mAmp for 1.5 hr (be sure the transfer gets to 300mAmp, may have to adjust voltage up to reach 280-300 mAmp mark)
III. Block
Carefully remove nitrocellulose membrane from red/black block
Cut membrane if necessary by staining to visualize desired band locations
Stain with 0.1% Ponceaus in 5% AAC (premade lab sln)
Wash excess stain off with dI water
Cut membrane at desired standard band
Rinse again in dI water (or TBS-T) to remove excess stain
Cover membrane with blocking solution and shake at RT for 1 hr
5ml for small container, 10ml for larger container
IV. Primary Anti-body
1. Pour off blocking buffer
2. Cover membrane thoroughly with TBS-T and place on shaker for 5 min. at RT
repeat for a total of 3 washes
Pour primary antibody solution over membrane*
a. add 1:1000 Dilution of p-4EBP1: 10 ml p-4EBP1 to 10 mL TBS-T
S
*see
antibody sheet to determine if dilute in 5% BSA
or milk in TBS-T
V. Secondary Antibody
1. Pour off and save primary antibody solution (store at 4)
2. Cover membrane thoroughly with TBS-T and place on shaker for 5 min. at RT
repeat for a total of 3 washes
3. Add secondary antibody and shake at RT for 1 hr
dilution of 1:20,000
a. Add 1.5uL anti-RABBIT secondary to 30 mL-T TBS
VI. Exposure
1. Discard secondary antibody
2. Cover membrane thoroughly with TBS-T and place on shaker for 5 min. at RT
repeat for a total of 3 washes
3. Add 4 ml SuperSignal West Pico luminal enhancer and 4 ml SuperSignal West
Pico stable peroxide solution to container and shake for 5 min. at RT
*if doing a large number of containers, mix the enhancer and peroxide
solution together just before adding to membranes
4. Remove membrane from solution, blot to dry, and wrap in plastic wrap
5. Expose membrane
1 BASICS OF THE THETA SYSTEM 1 2 VERB
1 CARACTERISTICAS BASICAS DE ESTILO DE UN INFORME EXPERIMENTAL
10 BASIC FUNCTIONS AND TRANSFORMATIONS NAME PD 1
Tags: buffer, 061008, p4ebp1, basic, western, solutions, running, protocol
- ZAVOD ZA ZDRAVSTVENO VARSTVO LJUBLJANA ENOTA ZASAVJE ZA
- UN VÍDEO SOBRE CONSEJOS EN LA CONDUCCIÓN Y LA
- DECRETO POR EL QUE SE CREA EL CONSEJO NACIONAL
- MINISTARSTVO GOSPODARSTVA RADA I PODUZETNIŠTVA OPERATIVNI PROGRAM POTPORA
- FACULTAD DE BIOANALISIS AGOSTO 2019 ENERO 2020 (2020 01)
- ENGLISH MACBETH TAKE HOME FINAL ESSAYS RATHER THAN A
- FIBROID EMBOLISATION (UAE) REFERRAL FORM DEPARTMENT OF RADIOLOGY GUYS
- D AUTOR KRZYSZTOF BARTECZKO ODATKOWE WYJAŚNIENIA DOTYCZĄCE KLAS
- ГОСУДАРСТВЕННЫЕ СТАНДАРТЫ СТАЛЬ КАЧЕСТВЕННАЯ И ВЫСОКОКАЧЕСТВЕННАЯ СОРТОВОЙ И ФАСОННЫЙ
- CURRICULUM VITAE NUME PRENUME DOBRE TEODORA LOCUL SI
- HE BARRIDO EL SUELO DE LA LUNA CON MIS
- KDBAREGULERINGSBESTEMMELSERR251 ÅS KOMMUNE PLAN NR R251 REGULERINGSBESTEMMELSER I TILKNYTNING
- 2 C FLORIDA INTERNATIONAL UNIVERSITY OFFICIAL UNIVERSITY PROCEDURE UNIVERSITY
- INFORMACJA DOT WYNAGRODZENIA Z TYTUŁU NIEOPŁACONEJ POMOCY PRAWNEJ UDZIELONEJ
- P ROGRESSION WITH SCRATCH A CTIVITIES TO BUILD COMPUTATIONAL
- STRENGTH OF MATERIALS MATH WORKSHEET ANSWERS 1 CALCULATE
- Artwork of Diana Magrann Diana Divides her Time Between
- METODISKAIS MATERIĀLS AR IEDZĪVOTĀJU IENĀKUMA NODOKLI NEAPLIEKAMIE IENĀKUMI 2018
- HABILIDADES SOCIALES PROGRAMA PARA MEJORAR LAS RELACIONES SOCIALES ENTRE
- UDRUGA ZAGREBAČKIH KOŠARKAŠKIH SUDACA STEGOVNIK NA TEMELJU ČLANKA
- ENCUESTA ABIERTA SOBRE EL MÉTODO DE APRENDIZAJE DEL PROYECTO
- THE STRESS STRAIN RELATIONSHIP FOR SOLIDS OPTI 521
- DELEGATURA PARA ASUNTOS JURISDICCIONALES GRUPO DE TRABAJO DE COMPETENCIA
- INTERNATIONAL GUIDEBOOK PREPARED FOR INTERNATIONAL STUDENTS AND VISITING SCHOLARS
- Town of Easton new Hampshire a Communication From the
- ARBEIDE I ITALIA NORSKE STATSBORGERE ER LIKESTILTE MED
- 102 AÑOS CUMPLIÓ NUESTRA QUERIDA ESCUELA JOSÉ MANUEL SALEDO
- 7 A KÖZIGAZGATÁSI ELJÁRÁSI TÖRVÉNY ÉS A TŰZVÉDELMI HATÓSÁGI
- UNIVERSITY OF ALBERTA REQUEST FOR ADMINISTRATIVE APPLICATIONS ACCESS REQUEST
- NAME PERIOD HEALTHY SNACK PROJECT ASSIGNMENT
 AUDICIÓN OBERTURA IMAGEN 1 SALÓN – CLARA Y JUAN
AUDICIÓN OBERTURA IMAGEN 1 SALÓN – CLARA Y JUAN COMITÉ OLÍMPICO COLOMBIANO INSTITUTO COLOMBIANO DEL DEPORTE INFORME TÉCNICO
COMITÉ OLÍMPICO COLOMBIANO INSTITUTO COLOMBIANO DEL DEPORTE INFORME TÉCNICOSB „MELIORATORIUS“ JURIDINIO ASMENS KODAS 30007 4473 BUVEINĖS ADRESAS
 CÓDIGO PLAZA ……………… RESOLUCIÓN FECHA ………… BOJA Nº DE
CÓDIGO PLAZA ……………… RESOLUCIÓN FECHA ………… BOJA Nº DECONSEJERÍA DE AGRICULTURA DESARROLLO RURAL MEDIO AMBIENTE Y ENERGÍA
ATTACHMENT 2 ITEM 3A1 NOVEMBER 21–22 2013 ELAELD SMC
 INFORMATION NOTICE NO 152007 WORLD INTELLECTUAL PROPERTY ORGANIZATION 34
INFORMATION NOTICE NO 152007 WORLD INTELLECTUAL PROPERTY ORGANIZATION 34 PC PASSPORT WORD PROCESSING STUDENT WORKBOOK PUBLISHED DATE
PC PASSPORT WORD PROCESSING STUDENT WORKBOOK PUBLISHED DATEGTBTNPHL162 PAGE 3 WORLD TRADE ORGANIZATION GTBTNPHL162 31 OCTOBER
 SIMBOLOGÍA ELÉCTRICA ÍNDICE 1 NORMA UNEEN 60617 (IEC 60617)
SIMBOLOGÍA ELÉCTRICA ÍNDICE 1 NORMA UNEEN 60617 (IEC 60617)!doctype Html Public w3cdtd Xhtml 10 Transitionalen !
REGISTRO DE REFERENCIA1 REGISTRO DEL ORGANISMO2 LOS CAMPOS MARCADOS
MENSAJE DEL PRINCIPAL ABRIL DE 2011 EDICIÓN ESPAÑOLA
2021 ÇAMAŞIR YIKAMA TEKNİK ŞARTNAMESİ TCDD TAŞIMACILIK AŞ İSTANBUL
ZAŁĄCZNIK NR 11 DO WNIOSKU WNW OŚWIADCZENIE DOTYCZĄCE ZAJMOWANEGO
LISTA DE ADMITIDOS ONARTUEN ZERRENDA «SKOLAE I TEORÍA
PEDIDO DE DEMISSÃO (TRABALHADOR DOMÉSTICO) À (NOME DO EMPREGADOR)
BCA UNDER CBCS WITH EFFECT FROM ACADEMIC YEAR 20162017
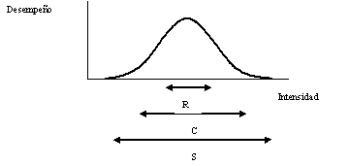 ECOLOGÍA GENERAL 2º CUATRIMESTRE 2017 APUNTES TEÓRICOS CONDICIONES Y
ECOLOGÍA GENERAL 2º CUATRIMESTRE 2017 APUNTES TEÓRICOS CONDICIONES Y CITTA’ EUROPEA DELLO SPORT NORME E CONDIZIONI DI CANDIDATURA
CITTA’ EUROPEA DELLO SPORT NORME E CONDIZIONI DI CANDIDATURA