ALLERGY IMPAIRMENT QUESTIONNAIRES VALIDATION STUDIES MARGARET C REILLY MA
ALLERGIES POLICY INCLUDING NUT & FOOD ALLERGY STATEMENT OFALLERGY IMPAIRMENT QUESTIONNAIRES VALIDATION STUDIES MARGARET C REILLY MA
ALLERGY PROVIDING ANTIGENIC HOMOGENEITY AND IDENTITY OF THE BODY
タガログ語版 アレルギー疾患調査票 SURVEY TUNGKOL SA SAKIT NA ALLERGY 年
CLINICAL IMMUNOLOGY AND ALLERGY SERVICE SHEFFIELD CLINICAL IMMUNOLOGY AND
CONTACT ALLERGY IN CHRONIC LEG ULCERS RESULTS OF A
ALLERGY IMPAIRMENT QUESTIONNAIRES: VALIDATION STUDIES
-
ALLERGY IMPAIRMENT QUESTIONNAIRES: VALIDATION STUDIES
Margaret C. Reilly, MA, MPH1; L. Ann Tanner, RPh, MPH2; and Eli O. Meltzer, MD3
1Reilly Associates, Waccabuc, New York, U.S.A.; 2Hoechst Marion Roussel, Kansas City, Missouri, U.S.A. and
3Allergy and Asthma Medical Group and Research Center, A.P.C., San Diego, California, U.S.A.
ABSTRACT
The work, activity, and classroom impairment of allergic rhinitis patients with moderate to severe allergy symptoms is described, testing the validity and responsiveness of allergy specific (AS) questions in the format of the Work Productivity and Activity Impairment questionnaire (WPAI; Reilly 1993). WPAI-AS questionnaires were completed by patients at baseline and after 1 and 2 weeks of treatment in 2 multicenter double-blind randomized placebo-controlled clinical trials of antihistamines: terfenadine (work/activity impairment; N=422) or fexofenadine HCl (classroom impairment; N=241). The validity, responsiveness (to clinical change), and reproducibility (in the absence of clinical change) of the WPAI-AS scores were measured utilizing symptom severity scores as the independent measures in the analysis. Allergy symptoms were associated with impairment at work, in the classroom, and in other regular daily activities. The studies established the discriminative and evaluative validity and the reproducibility of WPAI-AS measures of work impairment, overall work impairment (incorporates absenteeism into the impairment measure), classroom impairment, overall classroom impairment (incorporates absenteeism into the impairment measure), and daily activity impairment secondary to allergy symptoms. The measures of work and classroom time missed were not strongly associated with severity (or change in severity) of allergic rhinitis symptomatology. Calculations based on the underlying standard deviations were conducted to evaluate sample size needs for future studies. These results illustrate the magnitude of impairment secondary to moderate to severe allergic rhinitis symptoms in the workplace and the classroom. The studies also validate the use of the WPAI-AS as a tool in quality of life analysis of allergic rhinitis.
INTRODUCTION
Allergic rhinitis affects 10% to 20% of the U.S. population and is a major cause of absenteeism and loss of productivity in the workplace and the classroom. The measurement of these indirect costs in a clinical trial setting has previously been difficult.
The validity, responsiveness, and reproducibility of allergy-specific questions in the format of the Work Productivity and Activity Impairment questionnaire (WPAI-AS) scores were tested using clinical symptom severity scores as independent measures, and the work, activity, and classroom impairment of allergic rhinitis patients with moderate to severe allergy symptoms were described.
PATIENTS AND METHODS
The validation studies were conducted as part of two multicenter, randomized double-blind, parallel design clinical trials which assessed and compared the effects of various doses of a non-sedating antihistamine and placebo on the allergy symptoms of patients with moderate to severe allergic rhinitis. Patients included in the work/activity impairment study (study 1; N=422) were treated with terfenadine or placebo for two weeks during the 1993 fall allergy season. Patients included in the classroom impairment study (study 2; N=241) were treated with fexofenadine (MDL 16,455A) or placebo for two weeks during the 1994 spring allergy season. In both studies, patients in the intent-to-treat clinical dataset and voluntarily completing WPAI-AS at baseline, Week 1, and Week 2 were the subjects of the validation studies. Scores were calculated for baseline, Week 1, Week 2, and combined Week 1 and Week 2. Allergic rhinitis symptom severity scores were used as the independent measures in the testing of discriminative and evaluative validity, responsiveness, and reproducibility of the WPAI-AS.
Spearman's Correlation Coefficients were calculated between the impairment measures and average TSS (to test discriminative validity) and between the change in impairment measures and average change in TSS (to test evaluative validity). Linear regression was used to establish the relationship between the dependent measures (impairment) and the independent measure (average TSS) for discriminative validity and between the change in these measures for evaluative validity. In the responsiveness testing, linear regression was used to establish the relationship between the change in the dependent measures (impairment) and the independent measures (improvement classification; responder status). Covariates used in the regression models were demographics (age, race, gender, years of successive seasonal allergic rhinitis, and physical activity at work), baseline impairment scores, and treatment group (four active treatment groups vs. placebo).
RESULTS
Baseline Impairment Scores: Baseline TSS and WPAI-AS scores are listed in Table 1. Mean baseline TSS was higher for WPAI respondents in the Work/Activity study (8.2) than in the Classroom study (5.4). Average time missed from work was 1.7% of scheduled work hours, and average time missed from the classroom was 4.7% of hours usually in attendance. Average baseline impairment in work, activities other than work, and classroom ranged from 35% to 40%.
Discriminative Validity: All correlations between impairment measures and the corresponding average TSS scores were positive, indicating the symptom severity and impairment varied directly. In the regression analyses (Tables 2 and 3), low average TSS was the strongest and most consistent predictor (p=0.0001 or less) for lesser impairment at work and in the classroom (excluding absenteeism).
Evaluative Validity: For work/classroom impairment measures (other than absenteeism) all correlations were positive indicating changes in symptom severity and impairment varied directly. Average change in TSS was a significant predictor for change in all WPAI-AS measures except work/ classroom time missed. Greater improvement in Average TSS and higher baseline impairment scores were the strongest predictors for greater reductions in impairment measures at all intervals.
Responsiveness - Most Improved vs. Least Improved: The changes in the mean TSS and WPAI-AS scores for the 5% of patients with the greatest improvement in average TSS from baseline to combined Week 1 + 2 in the two studies are illustrated in Figures 1 and 2. For the most improved patients in both studies, mean TSS and impairment scores (except work time missed) decreased dramatically from baseline to follow-up assessments. For the least improved patients, mean scores for most measures generally increased (worsened) or stayed the same from baseline to follow-up assessments. A greater improvement in TSS scores was a significant predictor (p=0.0001 or less) for greater reductions in WPAI-AS measures, excluding work and classroom time missed.
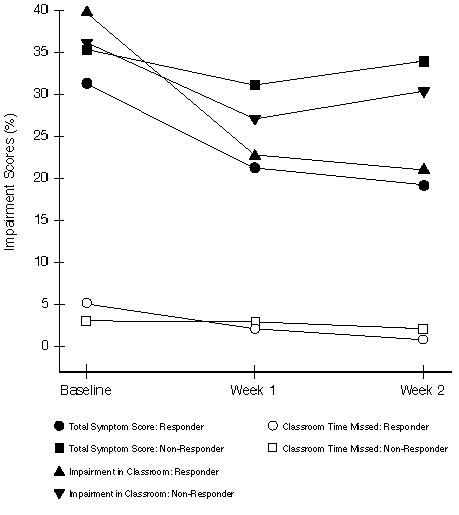
Responsiveness - Responders vs. Non-Responders Physician's Evaluation: The changes in mean TSS and WPAI-AS scores with time for patients classified as responders vs. non-responders, according to the Physician's Evaluation at Week 2, are illustrated in Figures 3 and 4. For responders in both studies, mean TSS and impairment scores decreased dramatically from baseline to follow-up assessments. For non-responders, scores decreased less or stayed the same. Classification as a responder by the Physician's Evaluation was a significant predictor (p=0.0001 or less) for greater reductions in WPAI-AS measures, except for work time missed and classroom time missed.
Reproducibility: There were no statistically significant differences between the Week 1 and Week 2 WPAI-AS scores (0.25 or less; or 0.5 or less change in average TSS) for patients with stable average TSS scores during the interval.
Sample Size Implications: To detect a 5% difference in impairment, with 80% power and 5% Type 1 error for a two-sided hypothesis test would require 201 patients/treatment group (work impairment) and 192 patients/treatment group (classroom impairment).

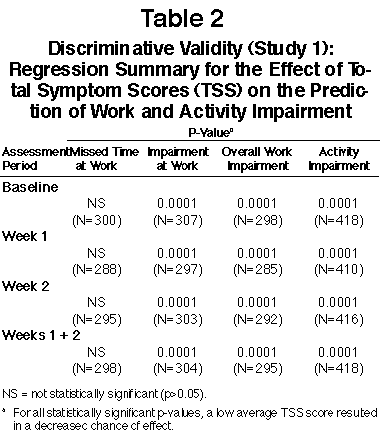

FIGURE 1
Responsiveness (Study 1): Average Total Symptom Scores (Expressed as Percentage of Total Possible Score) and Percentage Work and Activity Impairment Scores for the 5% of Patients Most Improved (N=21) and the 5% of Patients Least Improved (N=21) as Measured by Average Change in Total Symptom Scores from Baseline
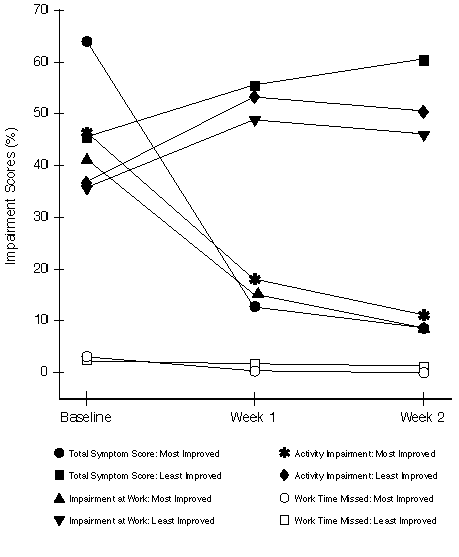
FIGURE 2
Responsiveness (Study 2): Average Total Symptom Scores (Expressed as Percentage of Total Possible Score) and Percentage Classroom Impairment for the 5% of Patients Most Improved (N=12) and the 5% of Patients Least Improved (N=12) as Measured by Average Change in Total Symptom Scores from Baseline

FIGURE 3
Responsiveness (Study 1): Average Total Symptom Scores (Expressed as Percentage of Total Possible Score) and Percentage Work and Activity Impairment for the Patients Considered as "Responders" (N=210) and "Non-Responders" (N=210) According to the Physician's Evaluation at Week 2
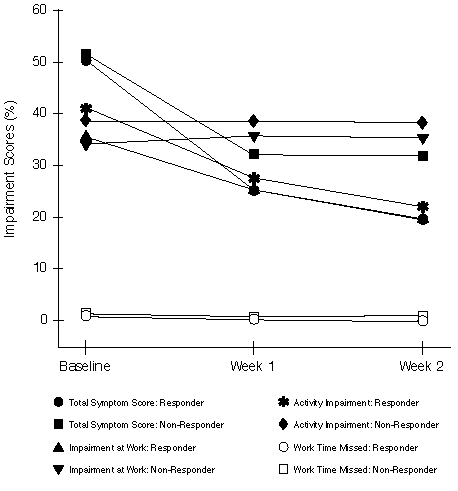
FIGURE 4
Responsiveness (Study 2): Average Total Symptom Scores (Expressed as Percentage of Total Possible Score) and Percentage Classroom Impairment for the Patients Considered as "Responders" (N=127) and "Non-Responders" (N=114) According to the Physician's Evaluation at Week 2
DISCUSSION
These studies established that moderate to severe allergy symptoms are associated with a high degree of impairment at work, at other regular activities, and in the classroom. While absenteeism was low (1.7% of work time and 4.7% of classroom time was missed), patient-reported impairment while at work or in the classroom ranged from 35% to 40% of normal productivity.
The discriminative and evaluative validity of all WPAI-AS measures (other than absenteeism) was established. For work impairment, overall work impairment (incorporates absenteeism into the impairment measure), activity impairment, classroom impairment, and overall classroom impairment (incorporates absenteeism into the impairment measure) at all follow-up intervals, TSS was a significant predictor (p=0.0001 or less) with lower TSS scores associated with lower impairment. Greater changes in TSS scores were strongly associated with greater changes in impairment. Similarly, the responsiveness of work impairment, overall work impairment, activity impairment, classroom impairment, and overall classroom impairment to clinically meaningful change was established using two separate comparisons. Finally, reproducibility of all WPAI-AS measures was established, by demonstrating a lack of change in WPAI-AS scores in patients with stable TSS. The measurement properties of the work/classroom time missed variables were not established because of infrequent absenteeism in the respective settings.
CONCLUSIONS
These results describe the work, activity, and classroom impairment of allergic rhinitis patients with moderate to severe allergy symptoms and validate the WPAI-AS as tools in quality of life analysis of allergic rhinitis. The results also provide data for selecting an adequate sample size to differentiate interventions in controlled studies measuring changes in work, activity, and classroom impairment secondary to allergic rhinitis symptoms.
REFERENCES
1. Meltzer E.O. An Overview of Current Pharmacotherapy in Perennial Rhinitis. J Allergy Clin Immunol 1995;5(Part 2):1097-1110.
2. U.S. Department of Health, Education, and Welfare (NIH). National Institute of Allergy and Infectious Diseases (NIAID), Task Force Report, Asthma and the other allergic diseases. Washington, D.C., 1979 (NIH) Publication No. 79-387.
3. Tarnsaky, Arsdel, Jr. Antihistamine Therapy in Allergic Rhinitis. J Fam Pract 1990;30(1):71.
4. McMenamin P. Costs of Hay Fever in the United States in 1990. Ann Allergy, 1994;73(1):35-39.
5. Meltzer E. Antihistamine- and Decongestant-Induced Performance Decrements. J Occup Med 1990 April;32(4):327-334.
6. Meltzer E. Comparative Safety of H1 Antihistamines. Ann Allergy, 1991 Dec 67:625-33.
7. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 1993;4(5):353-365.
8. Guyatt GH, Reeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med 1993;118:622-629.
9. Guyatt GH, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis 1987a;4:171-178.
Aventis
Pharmaceuticals U.S. Home
ORION
Home • ORION
Site Map
© 1999, Aventis Pharmaceuticals Inc.
CRAVEN COUNTY SCHOOLS EMERGENCY HEALTH CARE PLAN ALLERGY TO
FORM 4 SEVERE ALLERGYANAPHYLAXIS MANAGEMENT AND EMERGENCY RESPONSE
MENU ALLERGY ADVICE JANUARY 2015 BREAKFAST SHREDDED WHEAT CONTAINS
Tags: allergy impairment, of allergy, validation, allergy, margaret, impairment, questionnaires, studies, reilly
- ASEGURAMIENTO SANITARIO AUDITORIAS Y CERTIFICACIONES LISTA CHEQUEO SOLICITUD DE
- TEXAS COLONIAL WATERBIRD SOCIETY CONSTITUTION ARTICLE I – NAME
- – 3 – JANUARY 15 2022 BUREAU OF EPIDEMIOLOGY
- ANALISIS ISI KOLOM OPINI (STUDI KAJIAN DAKWAH DALAM KOLOM
- MCLEODUSA TELECOMMUNICATIONS SERVICES INC TARIFF TEXAS PUC NO 2
- FACULTYSTAFF BIOGRAPHIES 20172018 NOREEN BAGGETT FINANCE TEACHER – MRS
- COHERENCIA ESTRATÉGICA EN LA GERENCIA POR JORGE ENRIQUE VANEGAS
- YÖNETİM KURULU YILLIK ÇALIŞMA RAPORU A GENEL BİLGİLER
- CONVERGENCE LITERARY ART EXHIBITIONS CURATED BY CHRISTAMARIA LERM HAYES
- TALLER DE ESCRITURA ESCRIBIR UN RELATO DE VIAJE FICHA
- JOB DESCRIPTION JOB TITLE FUNDRAISING & MARKETING ASSISTANT
- INFORMACE O ÚČINNOSTI ZÁKONA Č 3002008 SB O ELEKTRONICKÝCH
- PSBKPPLSBK23 BAHAGIAN KESIHATAN PERGIGIAN NEGERI PERLIS BORANG KAJI SELIDIK
- FLEXIBLE WORKING POLICY INTRODUCTION THIS POLICY AIMS TO ENCOURAGE
- CONTRACTORS CHECK LIST FOR BUSINESS LICENSE 1 A CONTRACTOR’S
- THE SOCIETY OF EXPLORATION GEOPHYSICISTS MT EMAP DATA
- ARQUIVO MODELORESPOSTAPENDENCIADOC COEPUNIVATES VERSÃO OUTUBRO DE 2016
- ZAKON O CELOSTNI ZGODNJI OBRAVNAVI PREDŠOLSKIH OTROK S POSEBNIMI
- SEBÉSZI PRECIZITÁS MÉRNÖKI PONTOSSÁGGAL ORVOSOK MÉRNÖKÖK 3D TERVEZŐK ÉS
- KOKNESES NOVADA DOME KOKNESES MŪZIKAS SKOLA REĢ NR 457602256
- AKTIV UND PASSIV 1 BESTIMMEN IN DEN FOLGENDEN SÄTZEN
- 17 NEMZETI JOGVÉDŐ ALAPÍTVÁNY SZÉKHELY 3565 TISZALÚC KOSSUTH TÉR
- MODELO DE ESCRITO DE RECLAMACIÓN DE ANULACIÓN DE CLÁUSULA
- MEDELLÍN OCTUBRE 26 DE 2020 SEÑORES MINCIENCIAS AV CALLE
- DATA PRZEDMIOT NAUCZYCIEL TEMAT LEKCJI ORAZ FORMA PRACY UCZNIA
- PATVIRTINTA LIETUVOS MOKINIŲ NEFORMALIOJO ŠVIETIMO CENTRO DIREKTORIAUS 2016 M
- RAZLOMCI ZADACI S VIŠE RAČUNSKIH OPERACIJA I SA
- TALLER PLURALISMO CULTURAL MINORÍAS Y COOPERACIÓN SOLIDARIA CURSO ACADÉMICO
- ENTERING DATA COLLECTION SHEET INFORMATION INTRODUCTION WHEN ENABLED BY
- RESIDENCIA JOAQUÍN BLUME MADRID CAR MADRID REGLAMENTO
TOP OF FORM GUIDELINES FOR APPOINTMENTS TO POSITIONS NOT
LA SAGESSE RÉVÉLÉE DU DIEU VIVANT 1 «
THE DINOSAUR WHO LIVED IN MY BACKYARD QARTEACHER KEY
Ðïࡱáþÿ (¥áyà ð¿ú Bjbj½½ Ss{ß{úÿÿÿÿÿÿ·||öööööÿÿÿÿêêêê ö Ê¢øýýý!x9a²ltgöýýýýýgööû¡¡¡ýöö!¡ý!¡¡¡ÿÿÿÿpµò©ãx9fìÿÿÿÿÿ ¡ R0¢¡
UPUTA ZA PUŠTANJE U RAD RADIJSKIH POSTAJA KOJE SMIJU
 ORGANIGRAMA SCOLII GIMNAZIALE “TVSTEFANELLI” CAMPULUNG MOLDOVENESC A
ORGANIGRAMA SCOLII GIMNAZIALE “TVSTEFANELLI” CAMPULUNG MOLDOVENESC A PROTOKÓŁ Z POSIEDZENIA ZARZĄDU ZWIĄZKU GMIN I POWIATÓW SUBREGIONU
 ORDEN DE COMPRAVENTA DE DIVISAS A TRAVÉS DEL SISTEMA
ORDEN DE COMPRAVENTA DE DIVISAS A TRAVÉS DEL SISTEMA TRO OG LOVE ERKLÆRING IFØLGE LOV NR 1093 AF
TRO OG LOVE ERKLÆRING IFØLGE LOV NR 1093 AFDOCUMENTO 10 SITUACIÓN ACTUAL DE LA ESTRUCTURA PROCESO Y
 (VERSIÓN 1 ACTUALIZADA A FECHA 12112020) MODELO 2 SOLICITUD
(VERSIÓN 1 ACTUALIZADA A FECHA 12112020) MODELO 2 SOLICITUDKÉRELEM A KÖTELEZŐ ÓVODAI NEVELÉSBEN VALÓ RÉSZVÉTEL ALÓLI FELMENTÉSHEZ
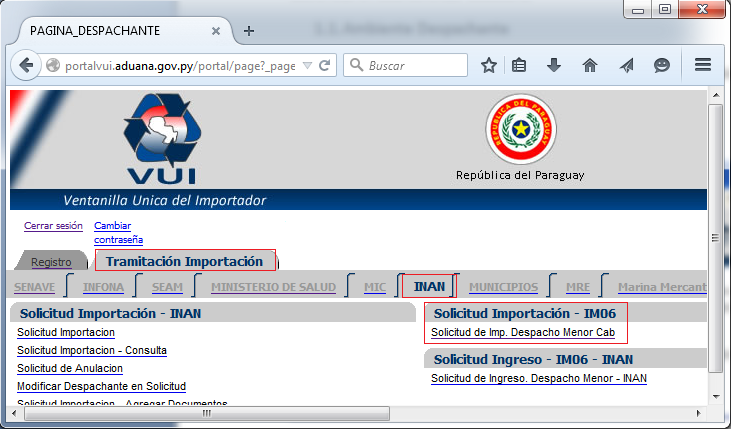 ÍNDICE 1 IMPORTACIÓN IM06 CABECERA 2 11 AMBIENTE
ÍNDICE 1 IMPORTACIÓN IM06 CABECERA 2 11 AMBIENTEZARZĄDZENIE REKTORA UNIWERSYTETU EKONOMICZNEGO W KRAKOWIE NR R0201162019 Z
REQUEST FOR NEW FEE TEXAS A&M UNIVERSITY – SAN
TEXAS EDUCATIONAL INSTITUTES – MARCH 30 2010 AEC TEXAS
 WZÓR UMOWA NR……………… O UDZIELENIE DOTACJI INWESTYCYJNEJ ORAZ WSPARCIA
WZÓR UMOWA NR……………… O UDZIELENIE DOTACJI INWESTYCYJNEJ ORAZ WSPARCIA X-203ManagerCorporateResearchPartnerships
X-203ManagerCorporateResearchPartnerships LOGO DE LA INSTITUCION CONTRAPARTE CONVENIO MARCO DE COOPERACIÓN
LOGO DE LA INSTITUCION CONTRAPARTE CONVENIO MARCO DE COOPERACIÓN FROM SHAKESPEARE FESTIVAL SHAKESPEARECUHKEDUHK SUBJECT EIGHTH CHINESE UNIVERSITIES SHAKESPEARE
FROM SHAKESPEARE FESTIVAL SHAKESPEARECUHKEDUHK SUBJECT EIGHTH CHINESE UNIVERSITIES SHAKESPEARE