KIM 110–2021 PRACTICE FINAL EXAM NAME AND SURNAME
KIM 110–2021 PRACTICE FINAL EXAM NAME AND SURNAME
1
Practice final exam
Name and Surname :
No :
Signature :
QUESTIONS
Substance A decomposes by a first-order reaction. Starting initially with [A] = 2.00 M, after 126 min [A] = 0.250 M. For this reaction, what is (a) t1/2 ; (b) k ?
T
he
initial rate of the reaction A + B C + D is determined
for different initial conditions, with the results listed in the
following table.
Expt [A],M [B],M Initial rate ( Ms-1)
1 0.185 0.133 3.35 x 10 -4
2 0.185 0.266 1.35 x 10 -3
3 0.370 0.133 6.75 x 10 -4
4 0.370 0.266 2.70 x 10 -3
What is the order of the reaction with respect to A and B ?
What is the overall reaction order ?
What is the value of the rate constant, k ?
W

hen
1.00 mol I2(g)
is introduced into an evacuated 1.00- L flask at 1200 oC,
5% of the I2
molecules dissociate into iodine atoms. For the reaction I2(g)
2I(g) at 1200 oC
What are the values of (a)
Kc
and (b)
Kp
?
Equilibrium is established at 1000 K, where Kc = 281 for the reaction
2

SO2(g)
+ O2(g)
2SO3(g).
The equilibrium amount of O2(g)
in 0.185-L flask is 0.00247 mol. What is the ratio of [SO2]
to [SO3]
in the equilibrium mixture?
What is the (a) degree of ionization and (b) percent ionization of propionic acid in a solution that is 0.45 M HC3H5O2 ?
H

C3H5O2
+ H2O
H3O+
+ C3H5O2-
pKa
= 4.89
6. Calculate the pH at the points in the titration of 25.0 mL of 0.132 M HNO2 when (a) 10.0
mL and (b) 20.0 mL of 0.116 M NaOH have been added. For HNO2, Ka = 7.2 x 10-4.
H
NO2
+ OH-
H2O
+ NO2-
7. Use VSEPR theory to predict the geometric shapes of the following molecules and
ions; (a) N2 , (b) HCN , (c) NO3-, (d) NSF.
8 Nicotine extracted from tobacco leaves, is a liquid completely miscible with water at
temperature below 60 oC. (a) What is the molality of nicotine in an aqueous solution that
starts to freeze at – 0.450 oC? (b) If this solution is obtained by dissolving 1.921 g of
nicotine in 48.92 g H2O, what must be the molar mass of nicotine? (Kf = 1.86 oC/m)
9. Tungsten(W) has a body-centered cubic crystal structure. Using a metallic radius of 139
pm for the W atom, calculate the density of tungsten.
10. The following first-order reaction occurs in CCl4 (l) at 45 oC.:
N
2O5
N2O4
+ ½ O2
(g). The rate constant is k
=
6.2 x 10-4
s-1.
An 80 g sample
of N2O5 in CCl4(l) is allowed to decompose at 45 oC.
How long does it take for the quantity of N2O5 to be reduced to 2.5 g ?
How many liters of O2, measured at 745 mmHg and 45 oC, are produced up to this point.
Tags: surname, 110–2021, practice, final
- THE STRUGGLE FOR ALIFE LIFE DON’T TALK TO
- LMNT VÄST RIKSFÖRENINGEN FÖR LÄRARE I MATEMATIK
- SAĞLIK BAKANLIĞI … HASTANESİ BAŞHEKİMLİĞİ’NE HASTANEMIZ … KLINIĞI’NDE …
- NUMBER AS91049 VERSION 4 PAGE 3 OF 3 ACHIEVEMENT
- FILIP GŁÓWKA NIEMIECKIE OBOZY PRACY PRZYMUSOWEJ W RADOMIU W
- 400001U DEPARTMENT OF EDUCATION NOTICE INVITING POSTSECONDARY EDUCATIONAL INSTITUTIONS
- !DOCTYPE HTML HTML DIRLTR LANGES XMLLANGES HEAD TITLECURSO PáGINA
- P R O J E K T UCHWAŁA SEJMU
- [DATAJPA76] AN INTERFACE LIKE THE JPAREPOSITORY WITH FINDER METHODS
- PROF DR SC IME PREZIME 4 SVEUČILIŠTE U SPLITU
- ISABEL ROMÁN CUADRILLERO COORDINADORA DE LOS SERVICIOS EN RELACIÓN
- WYKAZ SOŁTYSÓW GMINY OLESNO LP NAZWISKO I IMIĘ SOŁTYSA
- JUEGOS DE PRESENTACION Nº JUEGO RESUMEN PÁG PTOS ANTES
- GMINA JAKTORÓW – WYBORY WÓJTA NR NA LIŚCIE NAZWISKO
- B OTANIC GARDENS CONSERVATION INTERNATIONAL CONSERVATION CONCLUSIONS FROM THE
- MANEJO Y UTILIZACIÓN DEL PROGRAMA DE TRANSCRIPCIÓN QUICKBRAILLE 1
- C 05 HYPERPHOSTEMIA SERUM PHOSPHORUS AND MORTALITY IN
- ZAŁĄCZNIK 6A IDENTYFIKATOR SZKOŁY 2013
- SUBSCRIPTION RATES 2022 WIDENING PARTICIPATION AND LIFELONG LEARNING IS
- GOVERNO DO ESTADO DE SÃO PAULO SECRETARIA DE ESTADO
- PRESS RELEASE OVERVIEW OF ZUMTOBELS AUTUMN 2013 PRODUCT INNOVATIONS
- SPOROČILO ZA MEDIJE EKOSOCIALNA KMETIJA KORENIKA DOBRA PRAKSA TRAJNOSTNEGA
- HTTPZADANEPLZADANIE6545438 PILNE DAJĘ GNAJ ŹRÓDŁO DŹWIĘKU O CZĘSTOTLIWOŚCI 500
- FOMENTANDO COMPETENCIAS LITERARIAS PROPUESTA ESTRATÉGICA A PARTIR DE
- PCR230 MANUAL INTRODUCTION PCR230 IS A PROXIMITY READER
- WYKAZ ZDARZEŃ LOSOWYCH UWZGLĘDNIANYCH PRZEZ WYDZIAŁOWĄ KOMISJE STYPENDIALNĄ DO
- ENTRANT CONCURSANTE 1ST DRIVER PILOTO CODRIVER COPILOTO
- AN ORIGINAL METHOD OF EDGE DETECTION BASED ON CELLULAR
- F19 220514 REPUBLIKA SINGAPURU USTAWA O ZWIERZĘTACH I
- PAŃSTWOWY POWIATOWY INSPEKTOR SANITARNY W HRUBIESZOWIE 22500 HRUBIESZÓW UL
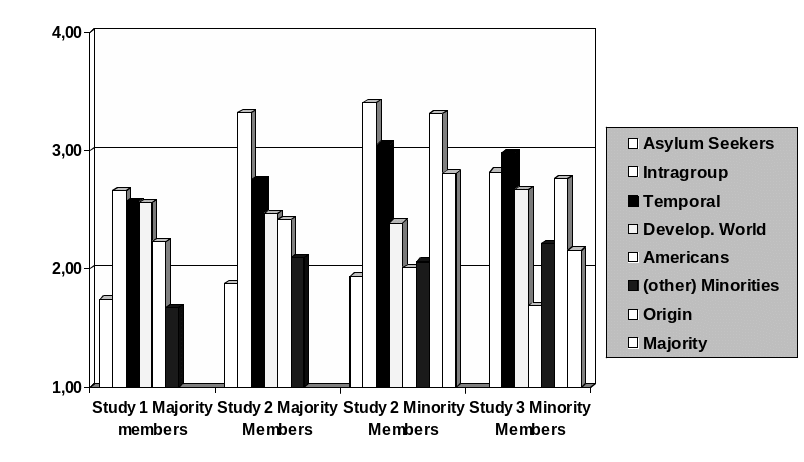 0 PREDICTING COMPARISON CHOICES IN INTERGROUP SETTINGS A NEW
0 PREDICTING COMPARISON CHOICES IN INTERGROUP SETTINGS A NEWCENTRUM NAUCZANIA W JĘZYKU ANGIELSKIM 60512 POZNAŃ UL JACKOWSKIEGO
INTEL® TEACH PROGRAM MENILAI PROYEK UJIAN AKHIR PERANG DUNIA
 DECLARACIÓN JURADA SOBRE LA PARTICIPACIÓN DE LOS INVESTIGADORES EN
DECLARACIÓN JURADA SOBRE LA PARTICIPACIÓN DE LOS INVESTIGADORES EN SERVICIO NACIONAL DE METEOROLOGÍA E HIDROLOGÍA DEL PERÚ SENAMHI
SERVICIO NACIONAL DE METEOROLOGÍA E HIDROLOGÍA DEL PERÚ SENAMHI KONKURSA NOLIKUMS BALTA GADA BALVA DROŠĀKAIS UZŅĒMUMA AUTOPARKS 2015
KONKURSA NOLIKUMS BALTA GADA BALVA DROŠĀKAIS UZŅĒMUMA AUTOPARKS 2015 EXAMEN TEMA 1 NÚMEROS REALES REPRESENTACIÓN DE NÚMEROS
EXAMEN TEMA 1 NÚMEROS REALES REPRESENTACIÓN DE NÚMEROSEL CAMBIO CULTURAL DE LAS NUEVAS FORMAS DIGITALES Y
ACCEPTANCE CRITERIA OF CONCRETE THE CLAUSE 16 (ACCEPTANCE CRITERIA)
GUIA DE PROBLEMAS DE FISICA III UNIDAD 6– MODELOS
 ÖNÉLETRAJZ BUCHHOLCZ NORBERT SZEMÉLYI ADATOK BUCHHOLCZ NORBERT M AGYARORSZÁG
ÖNÉLETRAJZ BUCHHOLCZ NORBERT SZEMÉLYI ADATOK BUCHHOLCZ NORBERT M AGYARORSZÁG UGC MEDICINA INTERNA HOSPITAL DE DÍA MÉDICO ESPECIALIZADO PLANTILLA
UGC MEDICINA INTERNA HOSPITAL DE DÍA MÉDICO ESPECIALIZADO PLANTILLA ELECTRONICA EJERCICIOS A CONTINUACIÓN SE PLANTEAN EJERCICIOS PARA QUE
ELECTRONICA EJERCICIOS A CONTINUACIÓN SE PLANTEAN EJERCICIOS PARA QUEOZNÁMENIE O KONANÍ KULTÚRNEJ ALEBO ŠPORTOVEJ AKCIE USPORIADATEĽ
DOPPLER EFFECT WEBSITE ACTIVITIES GO TO THE FOLLOWING WEBSITES
2 REUNIÓN ESPECIALIZADA DEL CIDI OEASER WII4
MINNEAPOLIS PUBLIC SCHOOLS (MPS) DEPARTMENT OF RESEARCH EVALUATION AND
 STUDIERETNINGSOPGAVEN (SRO) KØGE HANDELSGYMNASIUM SKRIVEVEJLEDNING TIL STUDIERETNINGSOPGAVEN PÅ KØGE
STUDIERETNINGSOPGAVEN (SRO) KØGE HANDELSGYMNASIUM SKRIVEVEJLEDNING TIL STUDIERETNINGSOPGAVEN PÅ KØGE ACIM,%20workbook%20TuTu
ACIM,%20workbook%20TuTu C OMPARER LES NOMBRES DÉCIMAUX REGARDE L’INÉGALITÉ QUI EST
C OMPARER LES NOMBRES DÉCIMAUX REGARDE L’INÉGALITÉ QUI EST