HACCP PLAN – FULLY COOKED NOT SHELFSTABLE WIENERS HAZARD
A MIKROBIOLÓGIAI KOCKÁZATBECSLÉS ÉS A HACCP RENDSZER KAPCSOLATA AEXEMPEL PÅ HACCPBASERADE FÖRFARANDEN ATT IDENTIFIERA DE FAROR
FACILITY HACCP TEAM FACILITY NAME DATE TEAM MEMBER NAME
FORMULÁŘ K VYPLNĚNÍ – PŘÍRUČKA HACCP PUBLIKACE ZÁSADY SPRÁVNÉ
FRÅGOR FÖR VALIDERING (UTVÄRDERING) AV HACCPPROGRAM 3 (3) MODELLBLANKETTER
HACCP PLAN – FULLY COOKED NOT SHELFSTABLE WIENERS HAZARD
HACCP Plan – Beef Slaughter
HACCP Plan – Fully cooked, not shelf-stable; Wieners
HAZARD ANALYSIS
|
1. Process Step |
2. Food Safety Hazard |
3. Reasonably likely to occur |
4. Basis of Reasonably likely to occur |
5. If Yes in Column 3, What Measures Could be Applied to Prevent, Eliminate, or Reduce the Hazard to an Acceptable Level? |
6. Critical Control Point |
|
1, Receiving and 6. Storage – Packaging materials |
Biological – Contamination with meat, other biological materials |
No |
Visual inspection for container integrity, contamination, at receiving makes hazard unlikely. SOP for storage makes hazard unlikely. |
|
|
|
Chemical – Non-food grade materials |
No |
Letters of guarantee are received from all suppliers of packaging materials. |
|
|
|
|
Physical – None |
No |
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
|
2. Receiving – Raw Meat/ Poultry and natural casings |
Biological Presence of pathogens: Salmonella, Listeria monocytogenes, Staphylococcus aureus, Clostridium perfringens, Clostridium botulinum; if beef E.coli 0157:H7, non-O157 Shiga-toxigenic E. coli (STEC); if poultry Campylobacter jejuni/coli.
BSE-causing prions |
Yes (pathogens)
No (prions) |
Raw meat/poultry is a known source of pathogens.
Letter of guarantee received from all suppliers of natural beef casings to certify that casings are not obtained from SRM (distal ileum). |
Hazard will be controlled by later CCP’s of cooking (destroys vegetative cells) and cooling/freezing (prevents germination and growth of clostridial spores). Letter of guarantee is on file for each supplier of ground or tenderized beef documenting the application of at least one intervention step against E. coli O157:H7. |
|
|
Chemical – None |
No |
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
|
Physical – None |
No |
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
|
3. Receiving – Food (Non-meat and Non-poultry) Ingredients: spices, cure mix, binders. |
Biological- None |
No
|
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
Chemical – Ingredients not being added or used as intended; ingredients containing undesirable substances |
No |
Approved formulations are followed; letter of guarantee are received from each supplier of food additives, spices, cure mix, binders, etc. |
|
|
|
|
Physical – None |
No |
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
|
4. Storage (Frozen or Refrigerated) – Raw Meat/Poultry |
Biological - Presence or growth of pathogens (see list in step 2 above) |
Yes (Presence)
No (Growth) |
Raw meat/poultry is a known source of pathogens.
Pathogens are unlikely to grow if raw meat/poultry is stored according to the SOP for storage. |
Hazard will be controlled by later CCP’s of cooking (destroys vegetative cells) and cooling/freezing (prevents germination and growth of clostridial spores). |
|
|
Chemical - None |
No |
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
|
Physical – None |
No |
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
|
5. Storage – Food Ingredients, both refrigerated and non-refrigerated |
Biological – None |
No |
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
Chemical – None |
No |
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
|
Physical – None |
No |
SOP for Receiving and Storage makes hazards unlikely to occur |
|
|
|
|
7. Tempering of raw meat/poultry |
Biological – Presence or growth of pathogens (see list in step 2 above) |
Yes (Presence) No (Growth) |
Raw meat/poultry is a known source of pathogens. Tempering done according to SOP for Tempering/Thawing of Frozen Materials. |
Hazard will be controlled by later CCP’s of cooking (destroys vegetative cells) and cooling/freezing (prevents germination and growth of clostridial spores). |
|
|
Chemical – None |
No |
SSOP makes hazards unlikely to occur |
|
|
|
|
Physical - None |
No |
SSOP makes hazards unlikely to occur |
|
|
|
|
8. Weighing of meat/poultry |
Biological - Presence or growth of pathogens (see list in step 2 above); contamination with pathogens via equipment (contaminated at start of shift) or workers |
Yes (Presence)
No (Growth)
No (Contamina-tion) |
Raw meat/poultry is a known source of pathogens. Weighing is done rapidly enough to prevent pathogen growth.
Pre-op and Operational SSOPs make hazard unlikely to occur |
Hazard will be controlled by later CCP’s of cooking (destroys vegetative cells) and cooling/freezing (prevents germination and growth of clostridial spores). |
|
|
Chemical – Cleaning/sanitizing chemical residues |
No |
Pre-op SSOP makes presence of chemical residues unlikely to occur. |
|
|
|
|
Physical - None |
No |
SSOP makes hazards unlikely to occur |
|
|
|
|
9. Grinding |
Biological – Pathogen (see list in step 2 above) contamination via equipment (contaminated at start of shift) and workers; presence of pathogens; growth of pathogens |
No (Contamina- tion)
Yes (Presence)
No (Growth) |
Pre-op and Operational SSOPs make hazard unlikely to occur.
Raw meat/poultry is a known source of pathogens.
Grinding is done rapidly enough to prevent pathogen growth. |
Hazard will be controlled by later CCP’s of cooking (destroys vegetative cells) and cooling/freezing (prevents germination and growth of clostridial spores). |
|
|
Chemical –cleaning/sanitizing chemical residues |
No |
Pre-op SSOP makes presence of chemical residues unlikely to occur. |
|
|
|
|
Physical – Metal |
No |
No history of problem (must provide evidence). Visual observation for foreign materials during processing, inspection of equipment during cleaning make hazard unlikely. |
|
|
|
|
10. Weighing of food ingredients |
Biological – Pathogen (see list in step 2 above) contamination via equipment and workers. Insufficient addition of cure mix.
|
No (contamina-tion)
No (cure)
|
SSOP makes contamination via equipment and workers unlikely to occur.
Finished product color would be unacceptable if insufficient cure added – product would not enter commerce. |
|
|
|
Chemical – Binders may contain potential allergens; cleaning/sanitizing chemical residues; excess cure addition |
No (allergens)
No (cleaning chemicals)
No (excess cure) |
Application of correct label prevents inadvertent consumption of allergen by consumer. Operational SSOP prevents cross-contamination of allergenic agents.
Pre-op SSOP makes presence of chemical residues unlikely to occur.
Cure mix has highly dilute concentration of nitrite, amount used is logged on batch sheet. |
|
|
|
|
Physical – None |
No |
|
|
|
|
|
11. Mixing |
Biological – Presence or growth of pathogens (see list in step 2 above); pathogen contamination via equipment (contaminated at start of shift) and workers |
Yes (Presence)
No (Growth)
No (Contamina- tion)
|
Raw meat/poultry is a known source of pathogens.
Mixing step is done rapidly enough to prevent pathogen growth.
SSOP makes contamination via equipment and workers unlikely to occur. |
Hazard will be controlled by later CCP’s of cooking (destroys vegetative cells) and cooling/freezing (prevents germination and growth of clostridial spores). |
|
|
Chemical – cleaning/sanitizing chemical residues |
No |
Pre-op SSOP makes presence of chemical residues unlikely to occur. |
|
|
|
|
Physical – Metal |
No |
No history of problem (must provide evidence). Visual observation for foreign materials during processing, inspection of equipment during cleaning make hazard unlikely. |
|
|
|
|
12. Chopping or Emulsifying |
Biological – Presence or growth of pathogens (see list in step 2 above); pathogen contamination via equipment (contaminated at start of shift) and workers |
Yes (Presence)
No (Growth)
No (contamina-tion)
|
Raw meat/poultry is a known source of pathogens.
Chopping or emulsifying is done rapidly enough to prevent pathogen growth.
SSOP makes contamination via equipment and workers unlikely to occur. |
Hazard will be controlled by later CCP’s of cooking (destroys vegetative cells) and cooling/freezing (prevents germination and growth of clostridial spores). |
|
|
Chemical – cleaning/sanitizing chemical residues |
No |
Pre-op SSOP makes presence of chemical residues unlikely to occur. |
|
|
|
|
Physical - Metal |
No |
No history of problem (must provide evidence). Visual observation for foreign materials during processing, inspection of equipment during cleaning make hazard unlikely. |
|
|
|
|
13. Stuffing |
Biological – Presence or growth of pathogens (see list in step 2 above); pathogen contamination via equipment (contaminated at start of shift) and workers |
Yes (Presence)
No (Growth)
No (contamina-tion)
|
Raw meat/poultry is a known source of pathogens.
Stuffing is done rapidly enough to prevent pathogen growth.
SSOP makes contamination via equipment and workers unlikely to occur. |
|
|
|
Chemical – cleaning/sanitizing chemical residues |
No |
Pre-op SSOP makes presence of chemical residues unlikely to occur. |
|
|
|
|
Physical - Metal |
No |
No history of problem (must provide evidence). Visual observation for foreign materials during processing, inspection of equipment during cleaning make hazard unlikely. |
|
|
|
|
14. Cooking |
Biological – Survival or growth of pathogens (see list in step 2 above)
|
Yes (presence)
No (growth) |
Raw meat/poultry is a known source of pathogens.
Cooking is done rapidly enough to prevent pathogen growth. |
Hazard will be controlled by exposing pathogens (vegetative cells, if present) to sufficiently high temperatures for a long enough time to ensure their destruction. |
1B |
|
Chemical – None |
No |
SSOP makes hazards unlikely to occur |
|
|
|
|
Physical - None |
No |
SSOP makes hazards unlikely to occur |
|
|
|
|
15. Cooling |
Biological – growth of Clostridium perfringens or Clostridium botulinum; presence or growth of Listeria monocytogenes or other pathogens (post-cook contaminants; see list in step 2 above). |
Yes (clostridial growth)
No (Presence of L.m. or other pathogens)
No (Growth of L.m. or other pathogens)
|
Spores survive cooking and can germinate and grow if cooling is done too slowly.
Listeria control measures, including testing program, make hazard unlikely. Pre-op and Operational SSOPs make contamination unlikely.
Product is refrigerated or frozen (minimizes or prevents pathogen growth)
|
Product is cooled rapidly enough to prevent growth of Clostridium perfringens (> 1 log) or Clostridium perfringens. |
2B |
|
Chemical – None |
No |
SSOP makes hazards unlikely to occur |
|
|
|
|
Physical - None |
No |
SSOP makes hazards unlikely to occur |
|
|
|
|
16. Packaging |
Biological - Growth of Clostridium perfringens or Clostridium botulinum; presence or growth of Listeria monocytogenes or other pathogens (post-cook contaminants). |
No (clostridial growth)
No (Presenc of L.m. or other pathogens)
No (Growth of L.m. or other pathogens)
|
Product does not get warm enough for a long enough time during packaging to result in growth.
Listeria control measures, including testing program, make hazard unlikely. Pre-op and Operational SSOPs make contamination unlikely.
Product is kept refrigerated/frozen (minimizes/prevents pathogen growth)
|
|
|
|
Chemical – improperly labeled allergens |
No |
Labeling SOP and SSOP make hazard unlikely to occur |
|
|
|
|
Physical - None |
No |
SSOP makes hazards unlikely to occur |
|
|
|
|
17. Finished product storage, shipping and/or retail sale |
Biological - None |
No |
Product is handled according to SOP for Finished Product Storage |
|
|
|
Chemical - None |
No |
Product is handled according to SOP for Finished Product Storage |
|
|
|
|
Physical - None |
No |
Product is handled according to SOP for Finished Product Storage |
|
|
05/11/2012 version; Supersedes all other versions
HACCP UTVRĐIVANJE I ANALIZA OPASNOSTI PRIJEDOLG PRIPREMA I POSLUŽIVANJE
haccp-washing-hands
HACCPBASED SOP LISTA DE VERIFICACIÓN DE SEGURIDAD ALIMENTARIA FECHA
Tags: wieners, hazard, cooked, shelfstable, fully, haccp
- 3 PALANGOS MIESTO SAVIVALDYBĖS KONTROLĖS IR AUDITO TARNYBA VIEŠAJAI
- FORMULAIRE D’ENREGISTREMENT ENTREPRISES DE DISTRIBUTION BASE LÉGALE ART 23
- TUSH PUSH 40 COUNT 4 WALL INTERMEDIATE CHOREOGRAPHER
- 14 OCTOBER 2016 SUBMISSION OF EFPIA COMMENTS ON GUIDELINE
- CASAL DE BARRI POU DE LA FIGUERA SOL·LICITUD D’ESPAI
- MOHAWK VALLEY COMMUNITY COLLEGE RESPIRATORY CARE PROGRAM COURSE OUTLINE
- NIEPAŃSTWOWA WYŻSZA SZKOŁA PEDAGOGICZNA W BIAŁYMSTOKU AL JANA PAWŁA
- TVARY MOLEKUL K OBJASNĚNÍ PROSTOROVÉHO ROZMÍSTĚNÍ ATOMŮ V
- MIGUEL LOYA DEL RÍO GARRIGUES ABOGADOS CONCLUSIONES DESPUÉS DE
- U MWELTMEDIZINISCHE BEGUTACHTUNG GEMÄSS NÖ HEILVORKOMMEN UND KURORTEGESETZ KURANSTALTENBEWILLIGUNG
- 0 GUIDANCE DOCUMENT MANAGEMENT AND DISPOSAL OF TREATED WOOD
- OZNACZENIE SPRAWY PUB252016DOPZ ZAŁĄCZNIK NR 1 – OPIS PRZEDMIOTU
- M UNICIPALIDAD PROVINCIAL DE ESPINAR C USCO PERU
- ŠTEVILKA 60341620201 DATUM 21 4 2020 NA PODLAGI 15
- CUESTIONARIO SOBRE LA AGENDA EUROPEA DE LA CULTURA EN
- MESTNA OBČINA KRANJ Ž U P A N SLOVENSKI
- PROPUNERI DE MODIFICARE SI COMPLETARE A LEGII DEŞEURILOR NR
- TERCERA CÁMARA LABORAL PODER JUDICIAL MENDOZA FICHA DE PRODUCCIÓN
- ÚLOHA AKO UVARIŤ PUDING? ROZBOR PROBLÉMU 1 ZA AKÝCH
- LORENTZ SUBMERSIBLE PUMP OPERATING MANUAL D&S REF MANUALIN27897 1
- UN DIENTE SANO ES UN DIENTE FELIZ UNA APLICACIÓN
- APAOC BYLAWS SEPTEMBER 201 ASIAN PACIFIC AMERICAN OFFICERS COMMITTEE
- CITY OF WESTMINSTER LHA SAFEGUARDS POLICY CONTENTS SECTION PAGE
- JÁSZNAGYKUNSZOLNOK MEGYEI HETÉNYI GÉZA KÓRHÁZRENDELŐINTÉZET KÖZPONTI RADIOLÓGIAI OSZTÁLY 5000
- TITLE 9 ENVIRONMENT STATE AIR POLLUTION CONTROL BOARD FINAL
- LEY DE LA JURISDICCIÓN CONSTITUCIONAL LEY DE LA JURISDICCIÓN
- NEWS RELEASE JANUARY 29 2009 FRESH START AT TDC
- HQ EC UNCLASSIFIED COMMERCIAL IN CONFIDENCE H EADQUARTERS EUROCORPS
- ASOCIACIÓN VECINAL 1 OBJETIVO EN LOS ÚLTIMOS AÑOS JUNTO
- ONTARIO REGULATION 15202 MADE UNDER THE HIGHWAY TRAFFIC ACT
 ARNO ARNOLD PI NEW CONCEPT FOR ROOF COVERINGS THE
ARNO ARNOLD PI NEW CONCEPT FOR ROOF COVERINGS THE OBČINSKA VOLILNA KOMISIJA OBČINE HAJDINA ZG HAJDINA
OBČINSKA VOLILNA KOMISIJA OBČINE HAJDINA ZG HAJDINASOUTH DAKOTA COALITION ENDING DOMESTIC & SEXUAL VIOLENCE BILL
 UNIVERSIDAD DE LAS PALMAS DE GRAN CANARIA GABINETE DE
UNIVERSIDAD DE LAS PALMAS DE GRAN CANARIA GABINETE DE CONSILIUL UNIUNII EUROPENE BRUXELLES 2 OCTOMBRIE 2012 (0410) (OR
CONSILIUL UNIUNII EUROPENE BRUXELLES 2 OCTOMBRIE 2012 (0410) (ORMODEL QUESTIONNAIRE TO BE COMPLETED BY PERSONS ALLEGING TORTURE
 NATIONAL SECURITY FRAMEWORK 31 SEARCHING OF THE PERSON THIS
NATIONAL SECURITY FRAMEWORK 31 SEARCHING OF THE PERSON THIS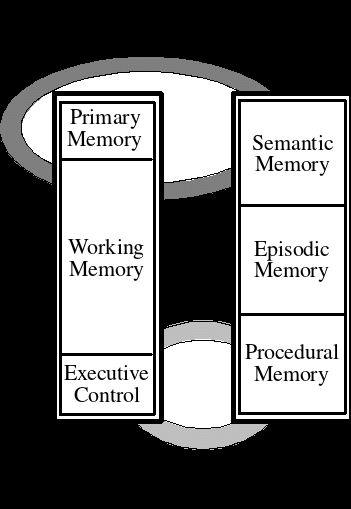 HUMAN FACTORS AND AGING IDENTIFYING AND COMPENSATING FOR AGERELATED
HUMAN FACTORS AND AGING IDENTIFYING AND COMPENSATING FOR AGERELATED F EDERACIÓN DE BANDAS DE MÚSICA DE LA REGIÓN
F EDERACIÓN DE BANDAS DE MÚSICA DE LA REGIÓNPART 1 SECTION 4 NINTH GRADE ENGLISH NAME
MINUTES OF REGULAR MEETING OF CITY COUNCIL CITY OF
 PORADNIK DLA OFIAR PRZEMOCY W RODZINIE (WG TERENOWEGO KOMITETU
PORADNIK DLA OFIAR PRZEMOCY W RODZINIE (WG TERENOWEGO KOMITETUPROGRAM SZKOLENIA I WYMAGANIA EGZAMINACYJNE NA PATENT STERNIKA MOTOROWODNEGO
NZQA UNIT STANDARD 28519 VERSION 2 PAGE 4 OF
Decreto 484 de 2007 (octubre 22) por el Cual
Silk Nonprofit law Counsel to Donors Foundations and Other
HTA PROGRAM SPECIALIST POSITION DESCRIPTION I IDENTIFYING INFORMATION POSITION
 ACTIVIDADES PARA REPASAR PARA LA PRUEBA EXTRAORDINARIA DE SEPTIEMBRE
ACTIVIDADES PARA REPASAR PARA LA PRUEBA EXTRAORDINARIA DE SEPTIEMBRE Samenvatting Titel Onverwachte Allergische Reacties op Voedsel bij Volwassen
Samenvatting Titel Onverwachte Allergische Reacties op Voedsel bij Volwassen KREIRANJE PAMETNE KUĆE SA KONTROLNOM FUNKCIJOM ZA OTVARANJEZATVARANJE PROZORAROLETNI
KREIRANJE PAMETNE KUĆE SA KONTROLNOM FUNKCIJOM ZA OTVARANJEZATVARANJE PROZORAROLETNI