ADVERSE EVENT TRACKING LOG STUDY TITLE PRINCIPAL INVESTIGATOR STUDY
Definitions 100 Series Avoid Adverse Impacts Means to0 REPORT ON ADVERSE DRUG REACTION 1 REPORTER (DOCTOR
A DVERSE EVENT NOTIFICATION FORM CHAPTER 7056 ADVERSE HEALTH
ADVERSE EVENT (AE) REPORTING FORM INSTITUTIONAL REVIEW BOARD (IRB)
ADVERSE EVENT FORM STUDY NAME SITE NUMBER PTID
ADVERSE EVENT REPORT SGU IRB APPLICATION REFERENCE ADVERSE EVENT
SERIOUS ADVERSE EVENT TRACKING LOG (SITE)
ADVERSE EVENT TRACKING LOG
|
Study Title: |
|
||
|
Principal Investigator: |
|
Study Coordinator: |
|
|
|
Subject ID |
Start Date of Event |
*Date Event Resolved |
Description of Event |
Severity of Event |
Nature of Event |
Relationship of Event |
Action with study drug 1- No Action 2- Interrupted 3- Discontinued |
Is this a UP involving risks to subjects or others? |
**Date Report Sent to HRPP |
|
1 |
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
|
* All events should be resolved or noted as unresolved at the time of subjects discontinuation in the study (i.e. study complete or subject withdrawal)
** Report all adverse events in accordance with HRPP Guidelines.
ADVERSE EVENT REPORTING FORM SECTION I INSTRUCTIONS FORMS
ADVERSE EVENT SUMMARY V 50 N (INCLUDE NUMBER
ADVERSE EVENT TRACKING LOG STUDY TITLE PRINCIPAL INVESTIGATOR STUDY
Tags: study title:, (i.e. study, study, adverse, tracking, event, investigator, principal, title
- OBOWIĄZEK INFORMACYJNY (PRZETWARZANIE DANYCH OSOBOWYCH ODBYWA SIĘ NA
- LECTURA EL JOROBADO Y OTROS CUENTOS DE “LAS MIL
- A THOUSAND TIMES CAPO 2 E A B7
- КОГДА СЛЕДУЕТ ОБРАТИТЬСЯ К ЛОГОПЕДУ? ЧТО ЯВЛЯЕТСЯ ПРИЧИНОЙ ПОСЕЩЕНИЯ
- UPUTE ZA KOLEKTIVNO OSTVARIVANJE AUTORSKIH PRAVA U AUDIOVIZUALNOM SEKTORU
- III JORNADAS DE DERMATOLOGIA ESTETICA Y ANTIENVEJECIMIENTO GRAN MELIÁ
- WILDCARD STUDIOS NEW PIERCING CARE SHEET
- INFORMATOR DLA ŚWIADCZENIODAWCÓW – ZASADY POSTĘPOWANIA Z OBCOKRAJOWCAMI KORZYSTAJĄCYMI
- VTIENCODINGSR|UTF8NL VTIAUTHORSR|PEDRO2XP\PEDRO VTIMODIFIEDBYSR|PEDRO2XP\PEDRO VTITIMELASTMODIFIEDTR|13 MAY 2014 100020 0000 VTITIMECREATEDTR|13
- V IAJE HASTA EL CORAZÓN DE LA TIERRA
- ZAMELDOWANIE DLA UCZESTNIKÓW ZAWODÓW WYPEŁNIĆ WYRAZNIE JEDNOZNACZNIE I W
- EURÓPAI PARLAMENT 2014 2019 COMMISSION{PETI}PETÍCIÓS BIZOTTSÁGCOMMISSION DATE{28022015}2822015DATE TITRETYPEKÖZLEMÉNY
- COLLEGE OF SCIENCE AND ENGINEERING POSTGRADUATE RESEARCH SCHOLARSHIP SCHEME
- ŠTEVILKA DOKUMENTA 00714620152 PRILOGE NAVODIL OU ZA POROČANJE IN
- M UNICIPALIDAD PROVINCIAL DEL CALLAO GERENCIA DE ABASTECIMIENTO RESUMEN
- COMPETENCIAS Y EVALUACIONES MASIVAS EN COLOMBIA UNA MIRADA
- ESPECIALIDAD EXCURSIONISMO REQUISITOS Y RESPUESTAS 1 DISCUTE CON
- PLANTILLA PARA LA REDACCIÓN DE CAPACIDADES PARA CARTA ESTA
- MỘT SỐ ĐIỂM MỚI VỀ QUYỀN CON NGƯỜI
- AUTORAUTORES TÍTULO CONGRESO LUGAR ORGANIZADOR FECHA PILAR SOLDEVILA Y
- SIMPLE HARMONIC MOTION LAB PART 1 HOOKE’S LAW
- PASCUA EASTER B 3 LECTURAS READINGS •
- HARMFUL ALGAL BLOOMS IN MALAYSIA OCCURRENCES AND MONITORING STRATEGIES
- INTERNATIONAL JOURNAL OF ENGINEERING APPLIED SCIENCES AND TECHNOLOGY 2015
- OPĆINA DONJI VIDOVEC RADE KONČARA 9 40327 DONJI VIDOVEC
- THIS PROCEDURE IS SUBJECT TO CHANGE WITHOUT WARNING! AUGUST
- CETTE FICHE A ÉTÉ ÉLABORÉE PAR PASCAL LENOIR
- COMMONLY USED OBJECT CODES A MORE DETAILED LISTING MAY
- 1474GINEBRA DE VENCI 1475ANUNCIACIÓN 1478BENOIS MADONNA 1481LA ADORACIÓN DE
- PIERWSZE POSIEDZENIE XLVII KADENCJI SENATU POLITECHNIKI WARSZAWSKIEJ W
OGLEDNI PRIMJERCI AKTA O OSNIVANJU I STATUTA ZAKLADE OGLEDNI
 OBRAZAC EN P R E P U B L
OBRAZAC EN P R E P U B L ROLNICTWO NA TERENACH WIEJSKICH WOJEWÓDZTWA ŻYJE BLISKO 998
ROLNICTWO NA TERENACH WIEJSKICH WOJEWÓDZTWA ŻYJE BLISKO 998COMITÊ DE PRONUNCIAMENTOS CONTÁBEIS PRONUNCIAMENTO TÉCNICO CPC 01 REDUÇÃO
ZAPISNIK OPERATIVNEGA SESTANKA – IZVEDBA PROJEKTA SLOVENSKE ČEBELARSKE ŠOLE
V SKLADU Z 61 ČLENOM KOLEKTIVNE POGODBE GRAFIČNE DEJAVNOSTI
TEKSTIN KIRJOITTAMINEN YKSI YKITESTIN NELJÄSTÄ OSAKOKEESTA ON KIRJOITTAMINEN (MUUT
University of Florida Radioactive Materials User Statement of Training
SCHEMA DE AJUTOR DE MINIMIS PREVĂZUTĂ ÎN CADRUL PROGRAMULUI
EL PROYECTO PERSONAL EL PORQUÉ DEL PROYECTO PERSONAL LA
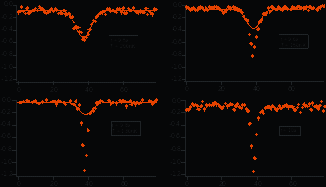 SYMPOSIUM LATSIS EPFL 2008 ALL OPTICAL PRODUCTION OF A
SYMPOSIUM LATSIS EPFL 2008 ALL OPTICAL PRODUCTION OF A U NIVERSIDAD DE CANTABRIA ESCUELA POLITÉCNICA DE INGENIERÍA DE
U NIVERSIDAD DE CANTABRIA ESCUELA POLITÉCNICA DE INGENIERÍA DE ALLEGATO I PUNTEGGI RIFERITI AGLI ELEMENTI DI VALUTAZIONE PUNTEGGI
ALLEGATO I PUNTEGGI RIFERITI AGLI ELEMENTI DI VALUTAZIONE PUNTEGGI EQUIPO DE SERVICIOS SOCIALES ÁREA (1605) DE VILLALBA DE
EQUIPO DE SERVICIOS SOCIALES ÁREA (1605) DE VILLALBA DETÚ LO SABES TODO DO FA SOL DO FA
6 APROBACIÓN INICIAL DE LAS TARIFAS DEL SERVICIO DE
PŘÍBALOVÁ INFORMACE INFORMACE PRO UŽIVATELE COLDASTOP RETINOLI PALMITAS
DESCENDIENTES DE PÁGINA 7 DE 7 RUFINA CHAVES ROJAS
ZÁPIS ZE SCHŮZE VÝBORU KSJU – ÚSTECKÉHO KRAJE ZE
PRAVILNIK 8 HRVATSKOG TRIENNALA AKVARELA 2019 GODINE 1 UVOD