IONIC AND METALLIC BONDING IONIC AND METALLIC SOLIDS ARE
POZIV NA RADIONICU “PRODAJA ZA PET” IZGRADITE SAMOPOUZDANJESCIENCE LESSON PLAN IONIC AND COVALENT BODING
1 NAMING BINARY IONIC COMPOUNDS IONIC COMPOUNDS ARE
7 THERMALLY STIMULATED PROCESS IN A CERAMIC IONIC CONDUCTOR
ACUTE TOXICITY OF VARIOUS NONIONIC SURFACTANTSSPREADERS USED WITH GLYPHOSATE
ADVENTSKA ČAROLIJA U VARAŽDINU PROSINAC 2013 KERAMEIKONOVE RADIONICE SRIJEDOM
General Aspects of Ionic Bonding: Lattice Energy
|
Ionic and Metallic Bonding |
Ionic and metallic solids are held together by strong Coulombic interactions on the order of the intramolecular bonding associated with covalent compounds and so we speak of ionic and metallic bonds. Strictly speaking, however, these “bonds” are not the same as those in covalent compounds which arise through the sharing of one or more pairs of valence electrons between atoms.
General Aspects of Ionic Bonding: Lattice Energy
Ions are held together in a crystal lattice by the attraction of ________________________ _________________. This 3-D array maximizes the attractive forces among cations and anions and minimizes the repulsive forces.
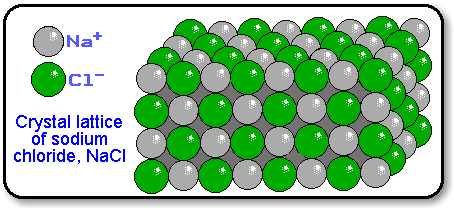
Lattice energy is the enthalpy change that occurs when _________________________
____________________________________________________. The size of the lattice energy can be likened to the _____________ of an ionic bond and depends primarily on two factors: ___________ _________ and ___________ ______________.
Lattice energy can be calculated using Coulomb’s Law. Coulomb’s Law for LiF would be stated as follows:
|
|
Where: QLi+ is _____________________________________
QF- is ______________________________________
r is _______________________________________
You will not need to be able to calculate lattice energy using Coulomb’s Law. What is important is that you notice that the charges are in the numerator and so lattice energy
________________________ as charge increases. The distance between the ions which increases with increasing ion size is in the denominator and so lattice energy ____________
as ionic size increases.
The size of the lattice energy value indicates the strength of the interaction between the ions and determines properties like _____________________________, _________________ and ___________________. Thus we can compare the melting point of two ionic compounds by comparing their ionic size and ionic charge.
For example as we go down a family in the periodic table ion size __________________ thus we would expect LiF to have a _____________________________________ and therefore _________________________ than NaF (LiF is about ____________ and NaF is about _______________). Similarly we would expect LiF to have a _________________
__________________________________ than LiCl (LiCl is about _________________).
If we look at two ionic compounds whose cations and anions are roughly the same size like LiF and MgO then charge becomes important. Since Mg2+ has _________________ the positive charge that Li+ has and O2- has __________________ the negative charge that F- has we would expect the lattice energy for MgO to be ___________________ than that of LiF (MgO is about ________ ).
Example:
Choose the compound with the higher lattice energy in each pair.
a) BaS and CsCl b) LiCl or CsCl
Choose the compound with the lower melting point in each pair.
a) CaS and BaS b) NaF and MgO
General Aspects of Metallic Bonding: The Mobile Sea of Electrons
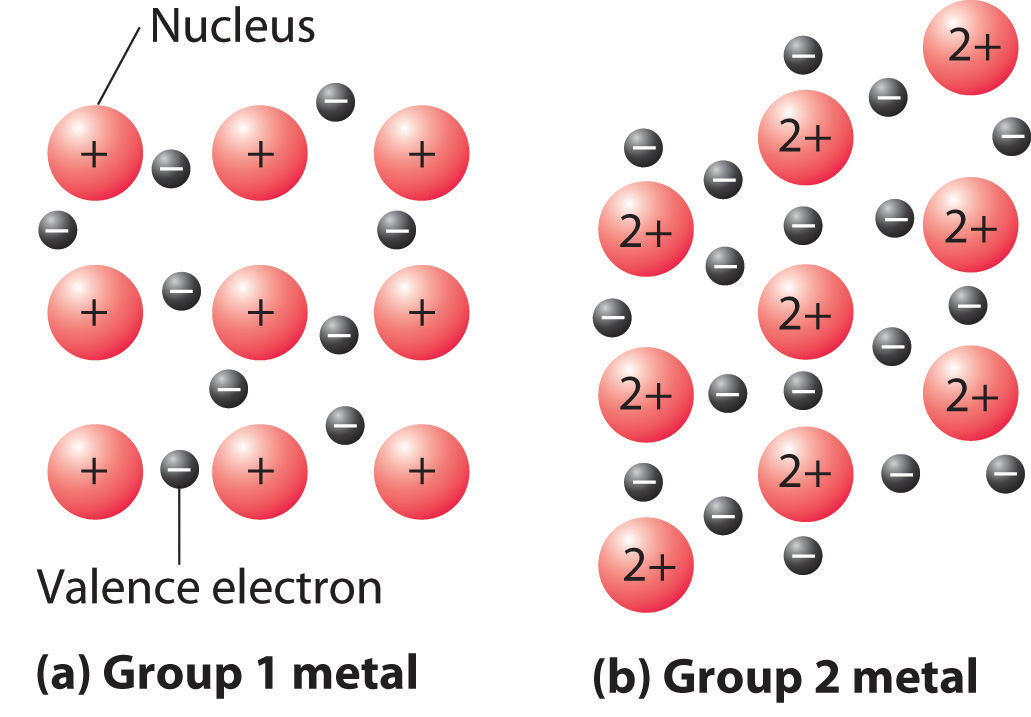
Metallic bonding describes an array of ________________________ charged metal cores composed of the ________________ and core ___________________, surrounded by a sea of mobile _____________________ electrons.
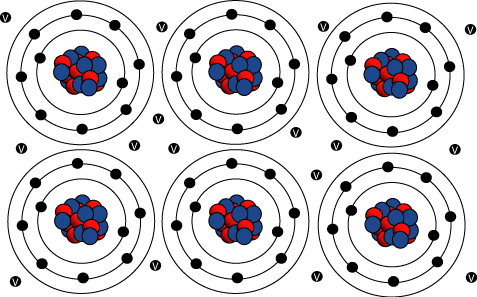
The more electrons in the mobile sea, the ______________________ the metal.
ALEXANDER KWARTIROFF TECHNOLOGY TEAM LEADER AVIONICS PARKER HANNIFIN ELECTRONIC
AP CHEMISTRY WORKSHEETMOLECULAR IONIC AND NET IONIC EQUATIONS 1
“EL POTENCIAL EFECTIVO DE UNA SOLUCION IONICA” H RUIZ
Tags: ionic and, two ionic, metallic, ionic, bonding, solids
- 1 EKİM 2010 TARİHİNDEN SONRA GENEL SAĞLIK SİGORTASI KANUNANA
- LOOKING FOR JOBS APPRENTICESHIPS (AUGUST 2011) 1 LOCAL
- DAVID BONNARDEAUX THE COCHABAMBA “WATER WAR” AN ANTIPRIVATISATION POSTER
- FACSIMILE DOMANDA DI PARTECIPAZIONE DIRECTOR OF THE DEPARTMENT OF
- PAQUETE DE ESTUDIO PARA CANDIDATOS AL SANTO MINISTERIO
- DIGITAL CODING (TECHNOLOGIES) – CREATING DIGITAL SOLUTIONS (F6)
- LIETUVOS RESPUBLIKOS APLINKOS APSAUGOS MINISTERIJOS IR LIETUVOS RESPUBLIKOS MIŠKŲ
- SECTION 32 32 19 MODULAR BLOCK RETAINING WALL 1
- OTROS PROYECTOS URBANIZACIÓN AÑO DENOMINACIÓN UBICACIÓN ESTADO 2005
- DECLARACION DE LOS ESTUDIANTES PARTICIPANTES EN EL I MOEA
- MAY 28 2009 1015 PM BAMBOOZLING OURSELVES (PART 2)
- WEST VIRGINIA FFA ASSOCIATION NOMINATING COMMITTEE PROCESS AND SUMMARY
- ENTITYANDATTRIBUTEINFORMATION DETAILEDDESCRIPTION ENTITYTYPE ENTITYTYPELABEL COMMODITYBYTRACTDBF ENTITYTYPEDEFINITION SHAPEFILE ATTRIBUTE TABLE
- 0 CHRONIC DISEASE PREVENTION & CONTROL IN THE AMERICAS
- ENTPEPARLAMENT EWROPEWENTPE 2004 2009 DATE{28032007}2832007DATE NODOCSE00342007NODOCSE TITRETYPEDIKJARAZZJONI BILMIKTUBTITRETYPE TITRERECUEILSKOND
- ARTISTS’ GARDEN COOPERATIVE ACTIVE ARTS CONFERENCE JULY &
- Whole Course Solutions What is a Whole Course Solution?
- STROKOVNO SREČANJE MANAGEMENT ZDRAVSTVENE NEGE DANES ZA JUTRI LJUBLJANA
- ANNEXE N° VII AGREMENTS DE LIGNE REGULIERE DELIVRES PAR
- PARTÍCULAS EN MOVIMIENTO TENEMOS UNA CAJA CON FORMA DE
- TERMO DE CONSENTIMENTO LIVRE E ESCLARECIDO – RELATO DE
- Case no Ar20050005816 Department of the Army Army Discharge
- HOSPITAL DE COQUIMBO SUBDIRECCION DE GESTION DE RECURSOS HUMANOS
- 10-14_congatec_certifies-conga-QA3_for_Intel_Gateway_Solution_for_IoT_eng_fp__Sp_
- VEHICLE DVR PRODUCT INSTRUCTIONS 48 ROAD 1080P HD
- SUPREME COURT OF THE UNITED STATES OCTOBER TERM 2011
- BUFFER SOLUTIONS DEFINITION A MIXTURE OF A WEAK ACID
- PROPOZYCJA SKŁADU KOMISJI EGZAMINÓW MAGISTERSKICH NA KIERUNKU PIELĘGNIARSTWO STUDIA
- 13 HUMAN RESOURCES MANUAL INSTRUCTION 3041 APPOINTMENT OF EXPERTS
- 4 PROJEKTŲ FINANSUOJAMŲ VALSTYBINIO VISUOMENĖS SVEIKATOS STIPRINIMO FONDO LĖŠOMIS
AMÉRICA LATINA EN EL SIGLO XXI INTRODUCCIÓN NUNCA
 MÓDULO 2 MECÁNICA DE SÓLIDOS Y FLUIDOS ÍNDICE PÁGINA
MÓDULO 2 MECÁNICA DE SÓLIDOS Y FLUIDOS ÍNDICE PÁGINA„A PTE UTAZÓ NAGYKÖVETE” TÁMOGATÁSI PROGRAM PÁLYÁZATI FELHÍVÁS 2019
 ST MATTHEW’S CE SCHOOL EAL (ENGLISH AS AN ADDITIONAL
ST MATTHEW’S CE SCHOOL EAL (ENGLISH AS AN ADDITIONALSPRING 2001 REHB 509B BEHAVIOR ANALYSIS RESEARCH DESIGNS GROUP
PRINCIPIOS PARA LA PROTECCIÓN DE LOS ENFERMOS MENTALES Y
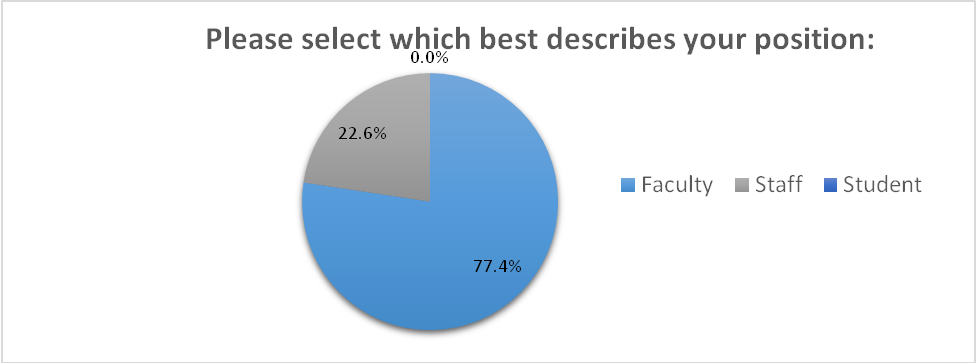 MEGAPLAN ACTIVITES SURVEY RESPONSE 04032017 SURVEY WAS ADMINISTERED 32817
MEGAPLAN ACTIVITES SURVEY RESPONSE 04032017 SURVEY WAS ADMINISTERED 32817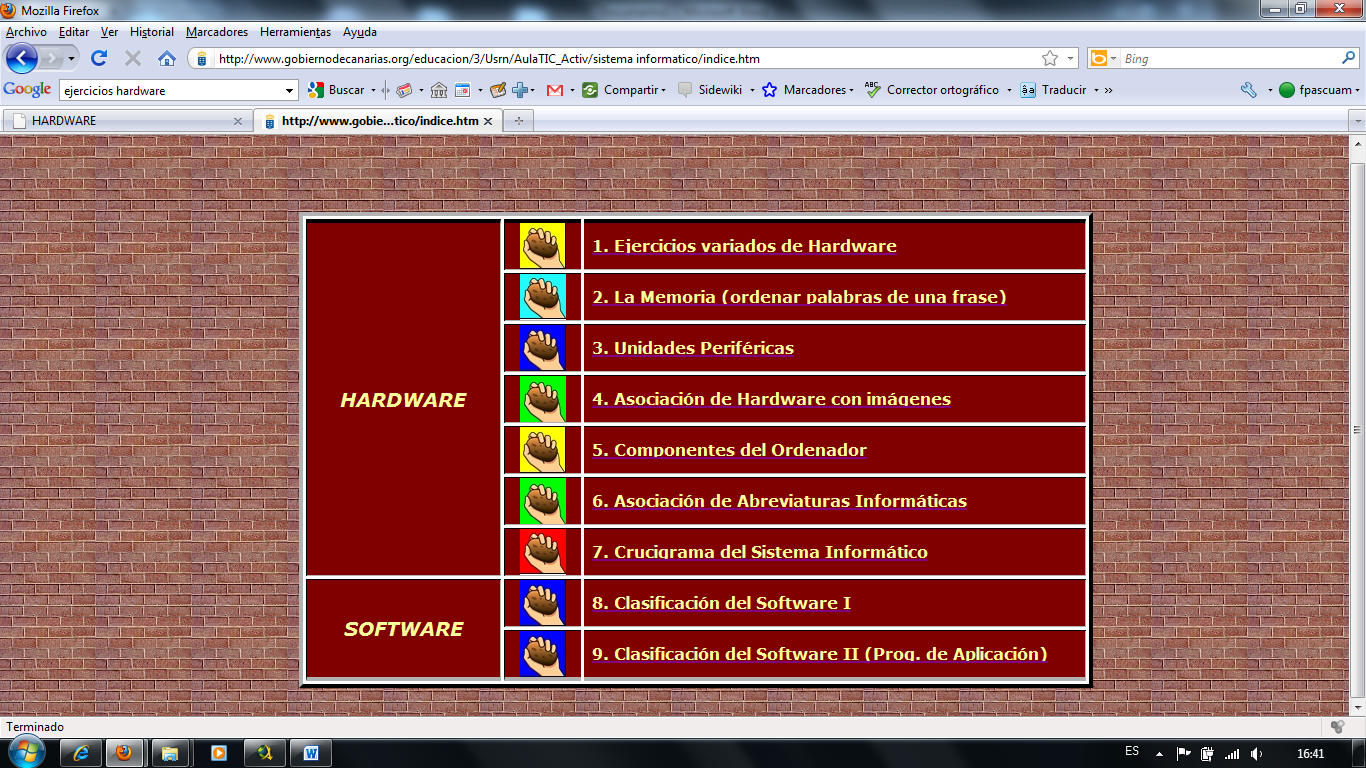 ACTIVIDAD 4 HARDWARE DEL PC REALIZAR LOS EJERCICIOS REFERENTES
ACTIVIDAD 4 HARDWARE DEL PC REALIZAR LOS EJERCICIOS REFERENTESNZQA UNIT STANDARD 30479 VERSION 1 PAGE 2 OF
NA TEMELJU ČLANKA 17 STAVAK 2 STATUTA TURISTIČKE ZAJEDNICE
 LESSON ELEMENT FILM EDITING INSTRUCTIONS AND ANSWERS
LESSON ELEMENT FILM EDITING INSTRUCTIONS AND ANSWERS6 BETEGTÁJÉKOZTATÓ INFORMÁCIÓK A FELHASZNÁLÓ SZÁMÁRA RUBOPHEN 500 MG
İLİMİZE 2017 YILI TEMMUZ AYINDA SÖZLEŞMELİ ÖĞRETMEN OLARAK ATAMASI
A GEOLOGIST’S LIFETIME LIST NAME DATE HOUR
 DIAGRAM PRACTICE BRACKET AND DIAGRAM THE FOLLOWING ARGUMENTS 1
DIAGRAM PRACTICE BRACKET AND DIAGRAM THE FOLLOWING ARGUMENTS 1MATA KULIAH KETERAMPILAN DASAR KEBIDANAN I KODE MK
NADAL L’ENCARNACIÓ DEL SENYOR PER SANT LLEÓ EL GRAN
DEKLARACJA PRZETARGOWA – WERSJA DO EDYCJI CZĘŚĆ DEKLARACJA PRZETARGOWA
LISTA KANDYDATÓW SPEŁNIAJĄCYCH WYMOGI FORMALNE URZAD GMINY PRZODKOWO UL
41 FLORENCE SAUNDERS BOOS CURRICULUM VITAE DEPARTMENT OF