COVALENT BOND CHARACTERISTICS 1) BONDS FORMED BY SHARING
SCIENCE LESSON PLAN IONIC AND COVALENT BODINGBKL CHAPTER 1 – LESSON 3 NOTES DEFINITION COVALENT
COMPARING THE SIMILARITIES AND DIFFERENCES OF IONIC VS COVALENT
COVALENT BOND CHARACTERISTICS 1) BONDS FORMED BY SHARING
HINTS FOR NAMING COVALENT COMPOUNDS COVALENT THEY ARE BOTH
IONIC AND COVALENT PROPERTIES LAB IN THIS LAB YOU
Ionic Bond Characteristics:
Covalent Bond Characteristics:
1.) Bonds formed by sharing outer s and p orbitals or sp3 orbitals
2.) Very directional atomic orbitals from directional molecular orbitals; non-spherical
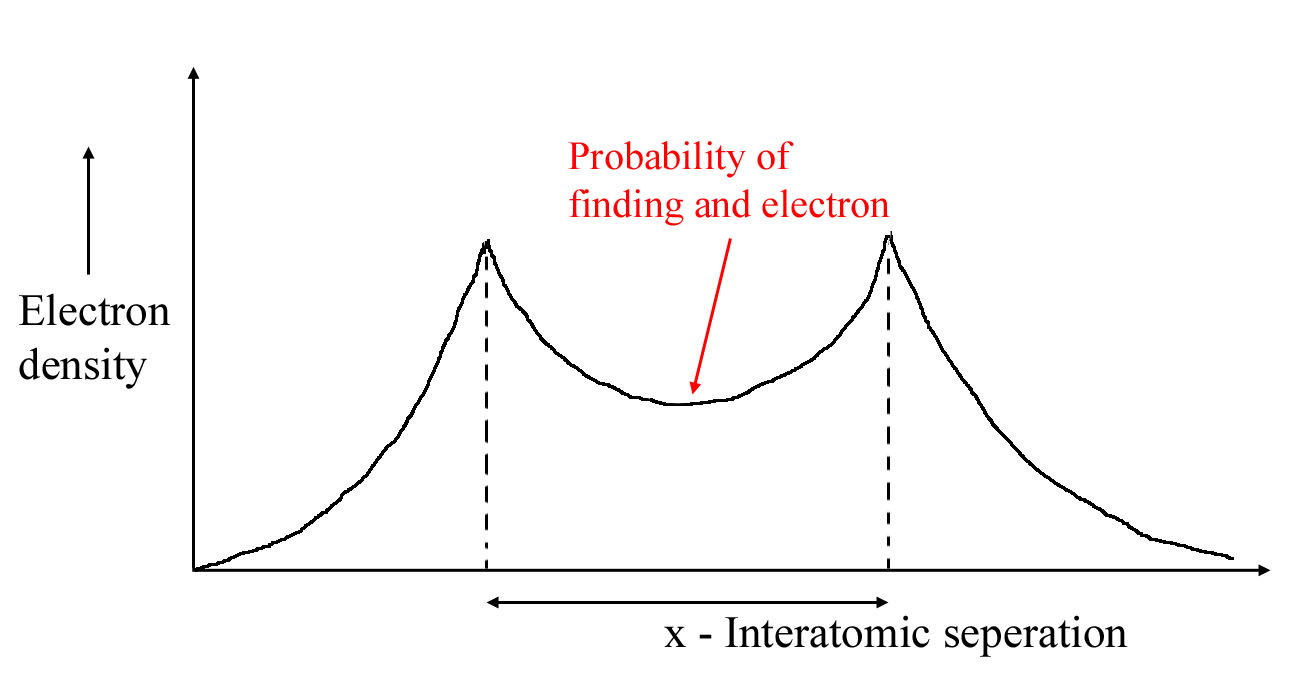
3.) High electron density between atom centers
4.) Electrons are held in narrow energy levels
- No conduction electrons (no free electrons; poor conductors of electricity)
- All valence electrons occupied in bonding (no free electrons)
5.) Coordination number fixed by number of bonding orbitals; shape of orbitals
6.) Neither atom gains or loses an electron
7.) Electrons are shared, where unfilled shells of one atom are filled by electrons of
another atom
8.) Diversity of polymers is due to covalent bonding
Ionic Bond Characteristics:
1.) Strong absorption in Infrared
2.) Transparent in visible spectrum
3.) High hardness, high strength, high melting point
4.) Energy lowering process by electron exchange
5.) Ionization to form complete outer shells (valence orbitals)
- Outer shells are spherical in nature and have noble gas configuration
6.) Coordination number controlled by geometry or radius ratio
7.) Leads to crystal structures – compounds not molecules
8.) Coulombic forces of attraction/repulsion determine bond length
9.) Hard sphere model (with oppositely charged spheres)
LAB DIFFERENCES IN IONIC AND COVALENT COMPOUNDS I PRELAB
LAB SHAPES OF COVALENT MOLECULES & POLARITY INTRODUCTION THE
METALLIC BONDING GIANT IONIC STRUCTURE SIMPLE COVALENT STRUCTURE GIANT
Tags: bonds formed, formed, characteristics, bonds, covalent, sharing
- 1984 ADVANCED PLACEMENT EXAM PART I MULTIPLE CHOICE NOTE
- EL PAPEL PREDOMINANTE DE LAS PYMES EN LA ECONOMIA
- ADJUST POWER ULTRASONIC CLEANER USER MANUAL ⊿ PLEASE READ
- EFFECTS OF COMMON AGRICULTURAL POLICY MEASURES ON INTRA AND
- ZAŁĄCZNIK 1A DO REGULAMINU PRZYZNAWANIA POMOCY MATERIALNEJ
- гͻÉÍ³Í 1 §¶É³ÓÁÑ ÀÔÌΦ Ö´À §´Ç½Ý»Ë ͳÑÏ»ÑÇ Ïѳٳ¹Ñٳݦ ÁÝóӳϳѷÇ
- G R A D S K I M
- INTERNET SADRŽAJ UVOD 1 RAZVOJ INTERNETA…………………………………………2 2 IZRAZ
- ARTIFAK FUNDACJA ARTIFAKT OGŁASZA KONKURS DLA DZIECI I MŁODZIEŻY
- 9 APRIL 2019 SUBMISSION OF COMMENTS ON CONCEPT PAPER
- IT360 SPRING 2008 LAB 5 SQL SOLUTION PART
- SUMMARY OF FINDINGS FROM THE REQUIREMENTS CHAPTER IN THE
- SMLOUVA O REALIZACI PROGRAMU CELOŽIVOTNÍHO VZDĚLÁVÁNÍ ČLÁNEK 1
- NEW JERSEY DEPARTMENT OF HUMAN SERVICES DIVISION OF AGING
- RS232 DB25 TO DB9 CONVERTER THE ORIGINAL PINOUT
- SOCIETATS CM005 SCM H 2060 ANTECEDENTS LES PUBLICACIONS PERIÒDIQUES
- GAIN REPORT CH5058 PAGE 8 OF 8 USDA
- PROGRAM OBRAZOVANJA ZA UČENJE CRNOGORSKOGA JEZIKA SADRŽAJ 1 NAZIV
- FLAT STANLEY MS MORROW’S 4TH GRADE MY TRIP TO
- LISTA NOMINOWANYCH W XVIII PLEBISCYCIE NA NAJLEPSZYCH SPORTOWCÓW WARSZAWY
- SADRŽAJ SADRŽAJ………………………………………………………1 UVOD…………………………………………………………2 TOKOVI NOVCA I KREDITA………………………………3 1NOVČANA MASA………………………
- Република Српска Министарство Рада и Борачкоинвалидске Заштите е Правилник
- ANEXA NR 181 A REGULAMENT CARTE FUNCIARĂ NR
- INNKALLING TIL ORDINÆRT SAMEIERMØTE 2010 ORDINÆRT SAMEIERMØTE I TOFTESGATE
- DJECA OŠTEĆENOG VIDA (SEMINARSKI RAD ) WWWMATURSKIORG SADRŽAJ UVOD………………………………………………………………………………………3
- ADAKAVO BENDRUOMENĖ 179727332 LIETUVOS KAIMO PLĖTROS 20072013
- ALEMÁN – 2º SESIÓN 34 (APUNTES DE LA
- BESTILLINGSSKJEMA FOR BLOKK A MALING AV DØRVINDUS KARMER
- ARAŞTIRMA GEMİLERİ SEFER SONUÇ RAPORU 1 GENEL BİLGİLER A
- NONPROFIT AND VOLUNTARY SECTOR QUARTERLY VOLUME 48 ISSUE 4
INSTRUCTION SHEET– DO NOT RECORD (DETACH THIS PAGE) THIS
ORDEN JUS…2012 DE … DE … POR LA QUE
THE US NATIONAL ARCHIVES AND RECORDS ADMINISTRATION HAS THE
 SBĚRNÝ DVŮR KLIKATÁ SBĚRNÝ DVŮR HL M PRAHY KLIKATÁ
SBĚRNÝ DVŮR KLIKATÁ SBĚRNÝ DVŮR HL M PRAHY KLIKATÁCERTIFICADO DE CIRCULACIÓN DE MERCANCÍAS 1 EXPORTADOR (NOMBRE APELLIDOS
 CARE AND PROTECTION WORKFORCE DEVELOPMENT A REPORT FROM THE
CARE AND PROTECTION WORKFORCE DEVELOPMENT A REPORT FROM THE GRAND VALLEY’S DANCE MARATHON 2009 DANCER REGISTRATION PACKET DEAR
GRAND VALLEY’S DANCE MARATHON 2009 DANCER REGISTRATION PACKET DEAR ZAŁĄCZNIK NR 1 (B) INSTRUKCJA SPORZĄDZANIA TRANSKRYPCJI RELACJI DO
ZAŁĄCZNIK NR 1 (B) INSTRUKCJA SPORZĄDZANIA TRANSKRYPCJI RELACJI DO CONSEJERIA DE EDUCACIÓN Y JUVENTUD SERVICIO DE EDUCACIÓN Y
CONSEJERIA DE EDUCACIÓN Y JUVENTUD SERVICIO DE EDUCACIÓN Y INCIDENT REPORT TO BE USED FOR INCIDENTS INVOLVING DOMESTIC
INCIDENT REPORT TO BE USED FOR INCIDENTS INVOLVING DOMESTICPITANJA ZA SREDNJE MEDICINSKE SESTRE(OPŠTI SMER) 01 ZADACI MEDICINSKE
 POWERPLUSWATERMARKOBJECT357831064 SAMPLE OFFER LETTER ADJUNCT APPOINTMENT MONTH DAY YEAR
POWERPLUSWATERMARKOBJECT357831064 SAMPLE OFFER LETTER ADJUNCT APPOINTMENT MONTH DAY YEARMODELO PARA REALIZAR UN COMENTARIO DE TEXTO HISTÓRICO UN
…………………………………… DNIA……… SĄD REJONOWY W WOŁOMINIE I WYDZIAŁ CYWILNY
SELECTION CRITERIA FOR EXTERNAL MANAGERS A USEFUL BYPRODUCT
 DEADLINE 530PM THURSDAY 8 APRIL 2021 DANCE AND THEATRE
DEADLINE 530PM THURSDAY 8 APRIL 2021 DANCE AND THEATRE MEVLANA DEĞİŞİM PROGRAMI KAPSAMINDA YÜKSEKÖĞRETİM KURUMLARINA AKTARILACAK TUTARLARIN KULLANIMI
MEVLANA DEĞİŞİM PROGRAMI KAPSAMINDA YÜKSEKÖĞRETİM KURUMLARINA AKTARILACAK TUTARLARIN KULLANIMI BUPATI LAMPUNG TENGAH PROVINSI LAMPUNG PERATURAN BUPATI LAMPUNG TENGAH
BUPATI LAMPUNG TENGAH PROVINSI LAMPUNG PERATURAN BUPATI LAMPUNG TENGAH OMB CONTROL NO 04120520 EXPIRATION DATE 3312021 CONTRACTOR EMPLOYEE
OMB CONTROL NO 04120520 EXPIRATION DATE 3312021 CONTRACTOR EMPLOYEEREAL DECRETO 18391997 DE 5 DE DICIEMBRE POR EL