POWERPLUSWATERMARKOBJECT3 3 9 ACADEMIC SCIENCE MONARCH PARK COLLEGIATE PARTICLE
POWERPLUSWATERMARKOBJECT3 PUBLIC HEALTH WALES UNSCHEDULED CARE0 PÁGINA 0 DE 1 POWERPLUSWATERMARKOBJECT3 CONVOCATORIA DE SUBVENCIONES
18 POWERPLUSWATERMARKOBJECT357831064 MENOPAUSE POLICY NHS HIGHLAND WARNING – DOCUMENT
2 PÁGINA 0 DE 2 POWERPLUSWATERMARKOBJECT3 CONCESIÓN DE SUBVENCIONES
24 POWERPLUSWATERMARKOBJECT357831064 DELTA COLLEGE FOUNDATION A CALIFORNIA NONPROFIT PUBLIC
3 POWERPLUSWATERMARKOBJECT357831064 JOB DESCRIPTION PERSON SPECIFICATION POST ALS ADMINISTRATOR
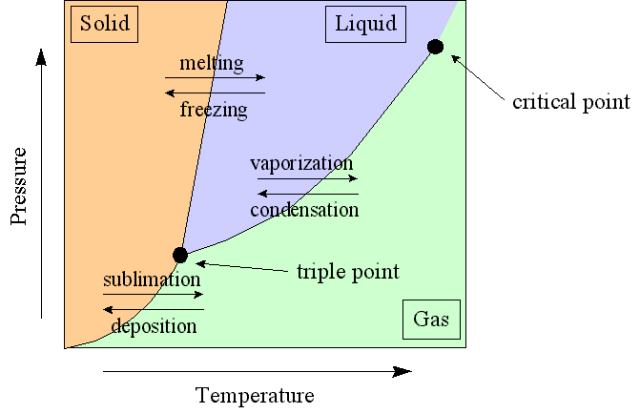
Particle Theory - Introduction
Particle Theory Of Matter – Introduction
The kinetic theory of matter (particle theory) says that all matter consists of many, very small particles which are constantly moving or in a continual state of motion. The degree to which the particles move is determined by the amount of energy they have and their relationship to other particles. The particles might be atoms, molecules or ions. Use of the general term 'particle' means the precise nature of the particles does not have to be specified.
Particle theory helps to explain properties and behaviour of materials by providing a model which enables us to visualize what is happening on a very small scale inside those materials. As a model it is useful because it appears to explain many phenomena but as with all models it does have limitations.
Solids, liquids and gases
|
In solids the particles |
In liquids the particles |
In gases the particles |
|
- are held tightly and packed fairly close together - they are strongly attracted to each other - are in fixed positions but they do vibrate |
- are fairly close together with some attraction between them - are able to move around in all directions but movement is limited by attractions between particles |
-have little attraction between them - are free to move in all directions and collide with each other and with the walls of a container and are widely spaced out |
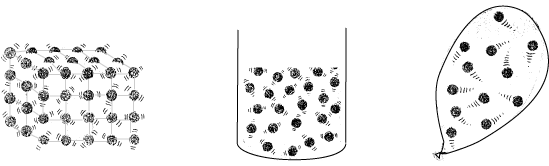
Fig 1 Particles in solids, liquids and gases
The model can be used to help explain the properties of matter and what happens during physical changes such as melting, boiling and evaporating
The properties of matter
|
Solids |
Liquids |
Gases |
|
- have a definite shape and maintain that shape - are difficult to compress as the particles are already packed closely together - are often dense as there are many particles packed closely together |
- do not have a definite shape - flow and fill the bottom of a container. They maintain the same volume unless the temperature changes - are difficult to compress because there are quite a lot of particles in a small volume - are often dense because there are quite a lot of particles in a small volume |
- do not have a definite shape - expand to fill any container - are easily compressed because there are only a few particles in a large volume - are often low density as there are not many particles in a large space |
Particle Theory of Matter S.T.A.M.P.
Spaces between the individual particles are very large compared to the sizes of the particles.
Together close, attract the most.
All particles of the same substance are identical to each other in every way. Different substances are made of different particles.
Matter is made up of small microscopic particles.
Particles of matter are always moving.
Changes of State (Phase Changes)
The particles of a solid or liquid possess kinetic energy (energy of motion) and potential energy. The potential energy is due to the force of attraction between the particles. To change the state of a substance, energy must be supplied or removed. For example, energy must be supplied to melt ice; energy must be removed from steam to change it into liquid water. If energy is supplied to a piece of ice which is at zero degrees Celsius it will cause the piece of ice to melt without causing it to change temperature. During this change of state, the energy supplied goes into increasing the total potential energy stored in the bonds between the molecules.
Similarly, during any change of state, it is the potential energy of the particles which changes; the average kinetic energy (and therefore the temperature) remains constant.
|
|
|
|
58TH CONFERENCE ON EXCEPTIONAL CHILDREN POWERPLUSWATERMARKOBJECT3 NC PT INSTITUTE
APPENDIX 3 POWERPLUSWATERMARKOBJECT357870517 CONFIDENTIAL JOB DESCRIPTION JOB TITLE EMPLOYER
ESTADÍSTICA I – PRÁCTICA 1 POWERPLUSWATERMARKOBJECT3 PRÁCTICA 2 ESTADÍSTICA
Tags: academic science, academic, science, particle, collegiate, powerpluswatermarkobject3, monarch
- MINISTÈRE DES AFFAIRES ÉTRANGÈRES N° 3 – ENERO DE
- SUSITIKIMAS SU RAŠYTOJA TATJANA PLATONOVA ASTROMINERALOGIJOS CENTRAS VILNIUS 2010
- 5 TEACHING VOCABULARY GOING BEYOND THE TEXTBOOK PENNY
- D OMOV DOMINO POSKYTOVATEL SOCIÁLNÍCH SLUŽEB ZAVIDOV 117 270
- SEA UN MONOPROCESADOR CON SISTEMA OPERATIVO DE PROPÓSITO GENERAL
- CONFERENCIA REGIONAL SOBRE MIGRACION CRM GRUPO REGIONAL DE CONSULTA
- CLUSTERING MAXIMO 71X WITH IBM WEBSPHERE 61 MULTIPLE APPLICATION
- MELDING ARBEIDSKRACHTEN VOOR REGELING INTERNATIONAAL HANDELSVERKEER WAAROM DIT FORMULIER?
- NOM COMERCIAL RAÓ SOCIAL NIFCIF ADREÇA DE L’ESTABLIMENT (NOM
- POSVET S SLOVENSKO CIVILNO DRUŽBO O POBUDI ZA REKOM
- UNIVERSIDAD DE BURGOS ESCUELA POLITÉCNICA SUPERIOR GRADO EN INGENIERÍA
- APPENDIX 2 USE AGREEMENT EMPLOYER TOP OF
- AUDIENCIA NACIONAL SALA DE LO PENAL SECCIÓN TERCERA ROLLO
- REGULAMIN WYDZIAŁU PRAWA I ADMINISTRACJI UNIWERSYTETU WARSZAWSKIEGO ROZDZIAŁ 1
- PRZEDSTAWIAM LICZBĘ MIEJSC NA KTÓRE MOGĄ APLIKOWAĆ STUDENCI UCZELNI
- 2 SPECIFIC AIMS THE STUDY OF WOMEN’S HEALTH ACROSS
- ARA JOB DESCRIPTION AND RESPONSIBILITIES POSITION TECHNOLOGY SERVICES JOB
- NA TEMELJU ČLANKA 53 I 65 ZAKONA O IZBORU
- MINUTES OF EASDEC BOARD MEETING 2016 CONFERENCE MEETING MANCHESTER
- SCT183 PROV PAGE 0 OMPI F SCT183 PROV ORIGINAL
- RFQ ATTACHMENT III CITY’S ADMINISTRATIVE REQUIREMENTS (LINKS TO FORMS)
- KALLELSE TILL SÖDERTELGE MÄSTERSKAPET 2006 SÖDERTELGE VOLLEYBOLLKLUBB VILL PÅ
- …………………………… MIEJSCOWOŚĆ DATA ……………………………… (PIECZĄTKA FIRMY) BURMISTRZ ŻABNA UL
- 2013 “PROYECTOS CLIMA” RESUMEN DE PROYECTO CÓDIGO DE PROYECTO
- 6 UNIVERSIDAD DEL ATLANTICO FACULTAD DE CIENCIAS JURIDICAS PROGRAMA
- Concurso Para la Vinculación de Profesores de Planta –
- PERGURUAN TINGGI MUHAMMADIYAH UNIVERSITAS AHMAD DAHLAN PROGRAM PASCASARJANA KAMPUS
- POLITECHNIKA WROCŁAWSKA WYDZIAŁ CHEMICZNY ZAKŁAD MATERIAŁÓW POLIMEROWYCH I WĘGLOWYCH
- TRANSFORMER® (ŠT ART1611T 1611T5) »IZBOLJŠEVALEC TAL« TRANSFORMER® IZBOLJŠA PRODIRANJE
- CARREGUEM UN DOCUMENT AL GOOGLE DOCS EN AQUEST DOCUMENT
 CHAPERONE POLICY RED ROOFS SURGERY IS COMMITTED TO PROVIDING
CHAPERONE POLICY RED ROOFS SURGERY IS COMMITTED TO PROVIDING VISITATIONSSKEMA INSTITUT FOR KOMMUNIKATION OG HANDICAP TJØRNEVEJ 6 8240
VISITATIONSSKEMA INSTITUT FOR KOMMUNIKATION OG HANDICAP TJØRNEVEJ 6 8240 Ficha de Datos de Seguridad Página 11 de 11
Ficha de Datos de Seguridad Página 11 de 11OBWODOWE KOMISJE WYBORCZE NA DZIEŃ WYBORÓW W DNIU 10
 Č ESKÉ POVSTÁNÍ PROTI HABSBURKŮM PO SMRTI RUDOLFA II
Č ESKÉ POVSTÁNÍ PROTI HABSBURKŮM PO SMRTI RUDOLFA II!doctype Html html Dirltr Langde head meta Charsetutf8 !
 MENJA MEDITERRANI MENJA SALUT LES UNIVERSITATS SALUDABLES FORMEN PART
MENJA MEDITERRANI MENJA SALUT LES UNIVERSITATS SALUDABLES FORMEN PART PROSTOWNIK JEDNO I DWUPOŁÓWKOWY STRONA 3 PROSTOWNIK JEDNO I
PROSTOWNIK JEDNO I DWUPOŁÓWKOWY STRONA 3 PROSTOWNIK JEDNO IKUNTERBUNT IST GOTTES GARTEN TEXT & MUSIK KURT
MALONOGOMETNA LIGA OPĆINE MARUŠEVEC PROLJECE 2016 2017 14
(……………… İHALEYI YAPAN MÜDÜRLÜĞÜN ADI YAZILACAKTIR) MALZEME DÂHİL YEMEK
0 PERSONAL AUTORIZADO A UTILIZAR Y ACCEDER EL SISTEMA
 CONTABILIDAD FINANCIERA TEMA 4 REPRESENTACIÓN CONTABLE LA
CONTABILIDAD FINANCIERA TEMA 4 REPRESENTACIÓN CONTABLE LA X COLOQUIO INTERNACIONAL TRADICIÓN Y MODERNIDAD EN EL MUNDO
X COLOQUIO INTERNACIONAL TRADICIÓN Y MODERNIDAD EN EL MUNDO PLAN POUR ATTEINDRE LE SITE « RUE BERTRAND »
PLAN POUR ATTEINDRE LE SITE « RUE BERTRAND »URBANISTIČKI PLAN UREĐENJA GOSPODARSKE ZONE STANČIĆ 1 2 I
 HR134 ACKNOWLEDGEMENT OF DEBT NOTE FORMS MUST BE
HR134 ACKNOWLEDGEMENT OF DEBT NOTE FORMS MUST BE ROZPORZĄDZENIE MINISTRA GOSPODARKI W SPRAWIE SZCZEGÓŁOWYCH WARUNKÓW FUNKCJONOWANIA SYSTEMU
ROZPORZĄDZENIE MINISTRA GOSPODARKI W SPRAWIE SZCZEGÓŁOWYCH WARUNKÓW FUNKCJONOWANIA SYSTEMUHTML HEAD TITLETHE BRITISH COLUMBIA FOREST SAFETY COUNIL
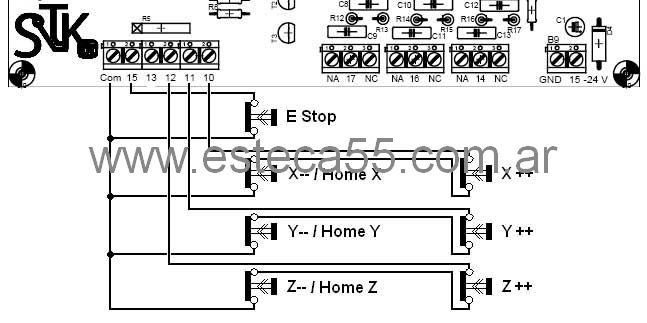 MACH 3 HOME & LIMIT EN ESTA OCASIÓN VOY
MACH 3 HOME & LIMIT EN ESTA OCASIÓN VOY