REIMBURSEMENTCOMPENSATION TO STUDY PARTICIPANTS PAGE 4 OF 4 COLUMBIA
REIMBURSEMENTCOMPENSATION TO STUDY PARTICIPANTS PAGE 4 OF 4 COLUMBIA
Human Subjects Reimbursement via Petty Cash Fund
Reimbursement/Compensation to Study Participants
Page
Columbia University Medical Center
Human Subjects Research
Reimbursement/Compensation to Study Participants
Scope of Policy:
This policy applies to all human research studies that will provide payments to participants or subjects for either: a) expenses that will incur as a result of participation (e.g., travel related costs); or b) payments to compensate subjects for their willingness to participate in the research study.
All plans to compensate participants/subjects in research studies must receive prior approval from the Institutional Review Board (IRB). All payments under $600 (total per year) to participants must be made via petty cash following the process outlined in section A of this policy. All payments over $600 per calendar year must follow the process outlined in section B of this policy.
Effective Date: April 12, 2005
Background:
Human research relies on volunteers to participate in studies. It is not uncommon for a researcher to reimburse subjects for travel or other expenses that they may incur as a result of participation in a study. Some studies may also offer compensation as a means to attract volunteers.
The IRB reviews all proposed plans for reimbursement or compensation provided to subjects to ensure that such payments are not coercive or provide the potential for undue influence. Another consideration of the IRB, particularly for research involving the collection of sensitive data, is that the confidentiality of the subject(s) is protected during the payment process if information about the subject must be forwarded outside the research team (e.g., to the Bursar’s Office).
Columbia University (CU) has developed this policy to provide a standard process to handle compensation of research subjects and to protect the confidentiality of subjects, to the extent allowable by law, during the reimbursement or compensation process.
A. PROCEDURES FOR PAYMENTS UNDER $600
All payments under $600 (total per year) to an individual subject must follow the following procedures, which allow payments to be processed without forwarding identifiable information about the participants to the Bursar’s (i.e. Business) Office.
Fund Establishment through AP-CAR
The departmental administrator will establish a unique petty cash fund for each project requiring human subject reimbursement/compensation. (For ease of management for multiple-year grants the initial fund can be opened against a non-grant account such as ledger 4 account - if funds allow).
The departmental administrator will submit a written request to the controller’s office (Chief Accountant) to justify the base amount of the fund as determined by: 1) the projected subject volume per week/month; 2) the projected reimbursement/compensation amount per subject; and 3) the projected time lag for fund replenishment. All other procedures for establishing a petty cash fund are followed (e.g. naming a fund custodian, obtaining Principal Investigator and Departmental approval via the Departmental Authorization Form, submitting a check request). The petty cash number assigned by the controller’s office is used as the fund identifier.
The fund custodian will cash the fund establishment check at the Bursar’s Office and will forward the petty cash to the study coordinator. A department may designate one individual to serve as both the study coordinator and the fund custodian.
Distribution of Petty Cash
Petty cash funds must be held in a secure location (a small safe is recommended) at the research site. The principal investigator or designated study coordinator is responsible for obtaining Columbia University research receipts with the following fields:
Copy 1 for subject (“Recipient” copy): Requisition Number (pre-printed on each research receipt), Date, Amount, Protocol Number, Participant Name*, Participant Signature*, Study Coordinator Name, and Study Coordinator Signature
Copy 2 for research site (“Project Coordinator” copy): Requisition Number (pre-printed on each research receipt), Date, Amount, Account Number, Protocol Number, Participant Name*, Participant Signature*, Study Coordinator Name and Study Coordinator Signature
*these items are considered confidential subject information and will not be forwarded to the controller’s office.
Copy 3 for fund custodian (“Controller’s Office” copy): Requisition #, Date, Amount, Account # (x-xxxxx-4910), Study Coordinator Name and Study Coordinator Signature
Research Receipt form copies:
Copy 1 will be given as a receipt to the study subject (optional);
Copy 2 must be kept at the research site as part of the permanent confidential records. These records must be maintained for purposes of financial audit;
Copy 3 will be retained by the study coordinator, temporarily, until they need to replenish the petty cash reserve. At that time, all copy 3 forms must be submitted to the fund custodian. When the fund custodian requests replenishment of the fund, he/she will turn in all “Copy 3” forms to the Bursar’s Office.
If it becomes evident that a subject will receive a series of payments that will result in total compensation greater than $600 in a calendar year then the departmental administrator must prepare a W-9 form (see B.2 below).
Fund Replenishment
The principal investigator or designated study coordinator will periodically (weekly/monthly) batch and tally up the Columbia University Research receipts and submit them to the fund custodian.
If using AP-CAR
The fund custodian will prepare a check request for the replenishment of the fund using the assigned petty cash number as the fund identifier and 4910 (human subjects) as the sub code. Copy 3 of the research receipts, which does not include confidential subject information, is attached to the check request as backup. All other procedures for preparing and submitting a check request are followed. The fund custodian cashes the fund replenishment check at the Bursar’s Office.
If using the Bursar’s Office
The fund custodian will prepare the petty cash reimbursement voucher and petty cash disbursement log for the replenishment of the fund using the assigned petty cash number as the fund identifier and 4910 (human subjects) as the sub code. Copy 3 of the research receipts, which does not include confidential subject information, will be attached as backup. All other procedures for petty cash disbursement through the bursar’s office will be followed.
Columbia University Research Receipt Forms
The Research Receipt Forms are available in the CUMC IRB office located in 722 W. 168th Street, Room 426. Both individual forms and booklets of forms are available.
B. Procedures for Payments $600 and Above
Compensation to study subjects of greater than $600 in a calendar year is considered taxable compensation and reportable to the Internal Revenue Service (IRS). Plans for protecting subject confidentiality in these cases will be reviewed by the IRB as part of the protocol/informed consent approval process.
If a
given payment is greater than $600, the departmental administrator
will:
enter a check request in AP-CAR providing the amount of the check as well as the name, address and social security number of the subject. An IRS Form 1099 will be issued to the recipient.
prepare a W-9 form providing the subject’s name, address, and social security number and submit it to the Controllers Office. A memorandum must accompany the W-9 form providing the amount of compensation to the subject. The memo and W-9 form must be mailed to:
AP Supervisor - 1099 Reporting
Columbia University Controllers Office
1700 Broadway, 11th floor
New York, NY 10019
If a series of payments results in total compensation greater than $600 to a subject in a calendar year, then the departmental administrator must complete step two above.
Informed Consent Requirements
The informed consent document, reinforced by the informed consent process, must clearly iterate the responsibility of the institution to report to the IRS as taxable income all payments to an individual subject aggregating $600 or more in a calendar year. The consent form must reflect the specific information being reported to the IRS (i.e., subject name, social security number, address, amount of payment). The IRB must review and approve the informed consent document prior to implementation and as stipulated in the informed consent policy.
Questions about the Policy
Any questions about the policy should be directed to the IRB Business Manager.
Tags: columbia university, reporting columbia, reimbursementcompensation, columbia, participants, study
- CURSO DE EDICIÓN DE IMÁGENES CON PHOTOSHOP CS4 INSTITUTO
- INSURANCE CERTIFICATE – CANCELLATION NOTIFICATION PROVISIONS MANY INSURANCE CERTIFICATES
- 1 ES UNIÓN EUROPEA COMITÉ DE LAS
- PERSONCENTERED PLANNING TRAINING MATERIAL FOR EMPLOYEES HIRED DIRECTLY BY
- WESTMINSTER MUSIC LIBRARY REGULATIONS FOR THE LOAN OF ORCHESTRAL
- USING IS ARE WAS AND WERE CORRECTLY – GROUPS
- 36 NÁVRH ZÁKON ZE DNE … 2013
- RALLEGRATEVI E SVEGLIATE IL MONDO1 CANTO INIZIALE PRIMA PARTE
- S AFETY BEST PRACTICES MANUAL CHAPTER 23 OSHA COMPLIANCE
- NOTRE RÉGIME DE RETRAITE ON SEN OCCUPE! LORS DE
- STRETCHED OFFER GUIDANCE A GUIDE TO STRETCHING THE EARLY
- CARRICKFERGUS RUGBY FOOTBALL CLUB FOUNDED 1865 AFFILIATED
- WEST VIRGINIA DEPARTMENT OF EDUCATION DIVISION OF TEACHING AND
- NERVOUS EVERYONE GETS NERVOUS WHEN YOU WALK INTO
- LIKOVNO IZRAŽAVANJE U PSIHOTERAPIJI SLIKA I CRTEŽ NUŽAN
- NASA FUTURE MISSIONS CGMS38 WHITE PAPER NOVEMBER 2010 BRIEF
- PROJET D’ARRÊTÉ PORTANT RENOUVELLEMENT DE TEMPS PARTIEL (À L’ISSUE
- I NDIANA SHERIFFS YOUTH LEADERSHIP CAMP 147 EAST MARYLAND
- INFORME DE VALORACIÓN DE LA PREDEFENSA DE TESIS DOCTORAL
- INSTITUCIJA KLASA URBROJ DATUM NA TEMELJU ODREDBE ČLANKA
- 262022 CONFERENCIA INTERNACIONAL DE PARLAMENTARIOS 2006 SOBRE LA IMPLEMENTACIÓN
- DEPARTMENT OF HEALTH AND HUMAN SERVICES MAINE CENTER FOR
- CITY OF LISMORE 249 EAST 2ND STREET●PO BOX 188●LISMORE
- MACROPROCESO DE GESTIÓN DE ATENCIÓN A GRUPOS DE INTERÉS
- LANESBORO COMMUNITY COLLEGE LIST OF BOOKS & EQUIPMENT FOR
- SOUTHERN CROSS UNIVERSITY LISMORE BAR THE NEW SOUTH WALES
- HTML WORKSHOPS FALL 2006–LIS WEB TEAM SESSION 3 INTRODUCTION
- BASIN BÜLTENI 09 EKIM 2017 43 YILLIK DENEYIMI YENILIKÇI
- Preparation of Reports in Modified Ieee Style Singlespaced one
- FORMAT — 2 C CHARTERED ACCOUNTANT’S CERTIFICATE (ON LETTER
THIS APP WORKS BEST WITH JAVASCRIPT ENABLED TITULACIONES
 CRITERIOS DE ADHESIÓN (CRITERIOS DE COPENHAGUE) TODO PAÍS QUE
CRITERIOS DE ADHESIÓN (CRITERIOS DE COPENHAGUE) TODO PAÍS QUE PROPRIETOR RESPONSIBILITY IT IS IMPORTANT THAT THE PROPRIETOR
PROPRIETOR RESPONSIBILITY IT IS IMPORTANT THAT THE PROPRIETOR TEN PLIK ZOSTAŁ UTWORZONY PRZY UŻYCIU ORACLE REPORTS PROSZĘ
TEN PLIK ZOSTAŁ UTWORZONY PRZY UŻYCIU ORACLE REPORTS PROSZĘ UNEPPOPSPOPRC21ADD1 NACIONESUNIDAS SC UNEPPOPSPOPRC21ADD1 PROGRAMA DE LAS NACIONES
UNEPPOPSPOPRC21ADD1 NACIONESUNIDAS SC UNEPPOPSPOPRC21ADD1 PROGRAMA DE LAS NACIONESTHE 2019 TYR SHAKER SHARK INVITATIONAL AGE GROUP &
 FORMULARIO OEA 608 (62001) SECRETARÍA GENERAL SUBSECRETARÍA DE
FORMULARIO OEA 608 (62001) SECRETARÍA GENERAL SUBSECRETARÍA DEPOR FAVOR RELLENE LOS DATOS PERSONALES EN MAYUSCULAS SOLICITUD
 RECYCLINGREFUSE INFORMATION THE VILLAGE OF WINNEBAGO HAS ONE WASTE
RECYCLINGREFUSE INFORMATION THE VILLAGE OF WINNEBAGO HAS ONE WASTETUTORETZA PLANA SARRERA 1TUTOREA ETA TUTORETZAREN ZEREGINAK TUTORETZA DA
PROVERBS INTRODUCTION THE WORD OF GOD CAME TO
 FORMULARIO DE PREINSCRIPCIÓN DATOS PERSONALES NOMBRE APELLIDOS DIRECC POSTAL
FORMULARIO DE PREINSCRIPCIÓN DATOS PERSONALES NOMBRE APELLIDOS DIRECC POSTALFY 2005 STATE LIST OF COUNTIES (AND NEW ENGLAND
HOW TO SUBSCRIBE TO THE TEACHING PROFESSOR 1) GO
1 A NAPRENDSZER TÉMA KÖVETELMÉNY 1 A NAPRENDSZERRŐL ÁLTALÁBAN
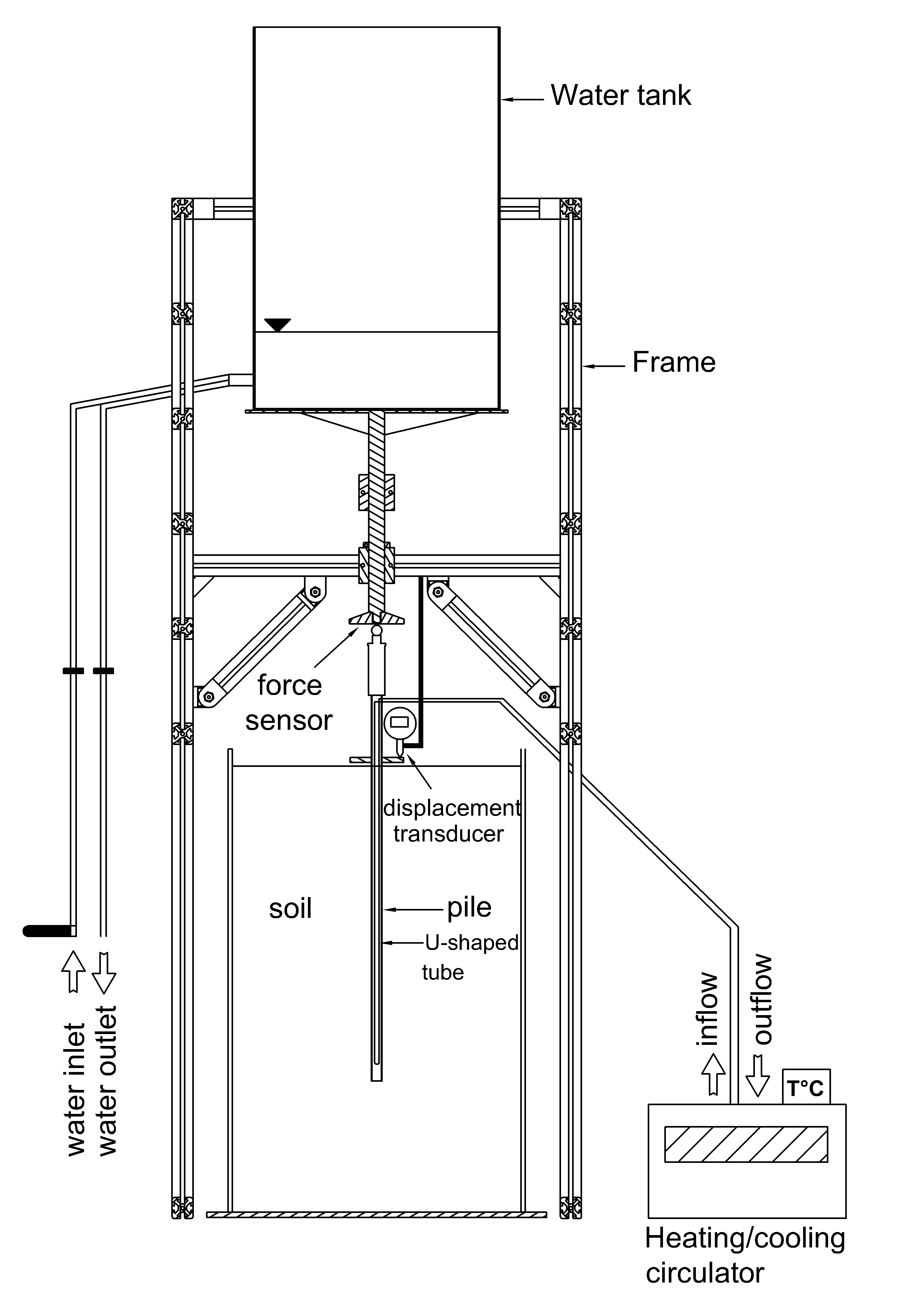 WRITTEN DATE AUGUST 26 2013 EXPERIMENTAL STUDY ON THE
WRITTEN DATE AUGUST 26 2013 EXPERIMENTAL STUDY ON THEHHA POLICY PERTAINING TO PAYMENTS TO BOARD MEMBERS INTRODUCTION
 Plant Disposal Specification Table Make Caterpillar Articulated Grader Model
Plant Disposal Specification Table Make Caterpillar Articulated Grader Model5 EMENTA AULA 17 19 DE MAIO DE
 ETS DE INGENIERÍA AGRONÓMICA ALIMENTARIA Y DE BIOSISTEMAS UNIVERSIDAD
ETS DE INGENIERÍA AGRONÓMICA ALIMENTARIA Y DE BIOSISTEMAS UNIVERSIDAD